Impact of Cyp 3a5 Genotype on Lcp Tacrolimus Dosing and Monitoring in Kidney Transplantation
Med Univ of South Carolina, Charleston, SC
Meeting: 2022 American Transplant Congress
Abstract number: 529
Keywords: African-American, Gene polymorphism, Kidney, Pharmacokinetics
Topic: Clinical Science » Pharmacy » 29 - Non-Organ Specific: Pharmacokinetics / Pharmacogenomics / Drug interactions
Session Information
Session Name: Non-Organ Specific: Pharmacokinetics / Pharmacogenomics / Drug interactions
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:00pm-6:10pm
Presentation Time: 6:00pm-6:10pm
Location: Hynes Room 311
*Purpose: LCP Tacrolimus has a recommended de novo starting dose of 0.14 mg/kg/day in kidney transplantation (KTR). There is limited data on the influence of various baseline or peri-operative factors on optimal doses needed to achieve steady-state LCP-Tac dosing, particularly CYP3A5 gene variants.
*Methods: This was a prospective observational study of adult KTRs receiving de novo LCP Tac oral therapy. After obtaining written consent, we measured CYP3A5 genotype allele status and assessed pharmacokinetic and clinical outcomes for 90-days post-transplant in an observational manner. Patients were classified as CYP3A5 expressors (*1 homo or heterozygous) or loss of function (LOF) [*3/*6/*7] allele. The primary study endpoints assessed stable LCP tac dosing across the two CYP3A5 groups, and if dose requirements, tac levels and monitoring were influenced by baseline or perioperative variables. Standard univariate and multivariable statistics were used to assess outcomes.
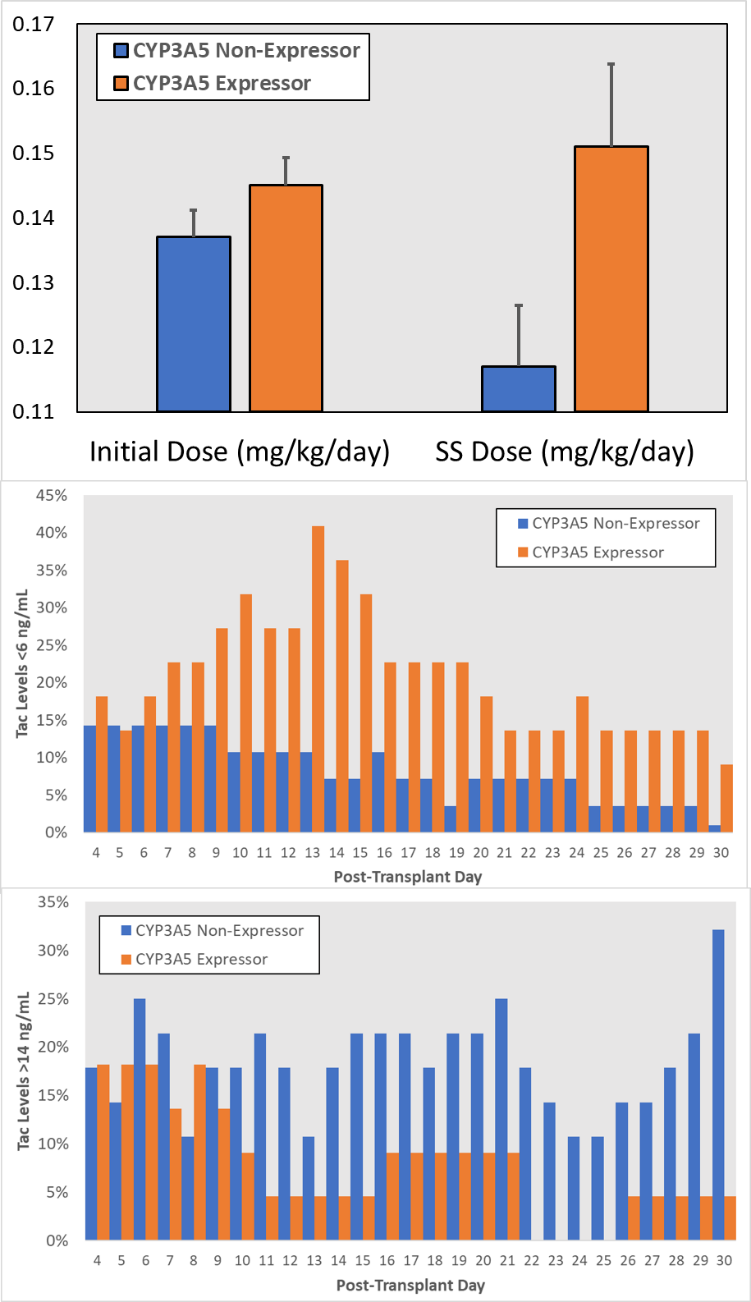
*Results: 120 patients were screened for inclusion, 90 were contacted and 52 provided consent. CYP3A5 genotype results were available on 50 patients; 22 patients (44%) were heterozygous (N=20) or homozygous (N=2) CYP 3A5*1 expressors. Genotype cohorts were well matched for baseline characteristics, except for AA race (35.7% of non-expressors were AA vs 81.8% expressors were AA; p=0.001). CYP3A5 expressors started with similar LCP-Tac doses (0.145 vs 0.137 mg/kg/day; p=0.161) while the steady-state (SS) LCP-Tac dose was significantly higher in the CYP3A5 *1 expressors (0.150 vs 0.117 mg/kg/day; p=0.026, Top Figure). CYP3A5 *1 expressors also had significantly higher numbers of tac levels <6 ng/mL, persisting 30-days post-transplant (Middle Figure; p<0.05) and significantly fewer tac levels >14 ng/mL, also persisting 30-days post-transplant (Bottom Figure; p<0.05). CYP3A5*1 expressors had 7.9 higher odds (p=0.039) of not having a therapeutic LCP-Tac level (6-10 ng/mL) within 10-days post-transplant. In sequential models, the impact of AA race on LCP-Tac dose and subtherapeutic Tac levels was completely abrogated after controlling for CYP3A5*1 expression.
*Conclusions: CYP3A5*1 expressors require higher doses of LCP Tac to achieve therapeutic levels; Expressors are at higher risk of sub-therapeutic levels that persisted for at least 30-days post-transplant and CYP3A5*1 genotype abrogates the influence of race of LCP Tac dosing requirements. Prospective CYP3A5 gene variant measurement may help improve immediate post-transplant dosing and monitoring for LCP Tac.
To cite this abstract in AMA style:
Taber DJ, Carcella T, Patel N, Bartlett F, Posadas A, Rao V, Casey M, Rohan V, Dubay D, Taber D. Impact of Cyp 3a5 Genotype on Lcp Tacrolimus Dosing and Monitoring in Kidney Transplantation [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-cyp-3a5-genotype-on-lcp-tacrolimus-dosing-and-monitoring-in-kidney-transplantation/. Accessed March 2, 2026.« Back to 2022 American Transplant Congress