Assessing Safety of Using ECMO-Supported Donors in Abdominal Transplantation
1Department of Surgery, Thomas Jefferson University, Philadelphia, PA, 2Gift Of Life Donor Program, Philadelphia, PA
Meeting: 2022 American Transplant Congress
Abstract number: 437
Keywords: Donors, marginal, Kidney transplantation, Liver transplantation, Organ Selection/Allocation
Topic: Clinical Science » Organ Inclusive » 68 - Deceased Donor Management and Intervention Research
Session Information
Session Name: Surgical Issues: Donor and Recipient
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:40pm-3:50pm
Presentation Time: 3:40pm-3:50pm
Location: Hynes Room 310
*Purpose: Organ donors supported by extracorporeal membrane oxygenation (ECMO) are historically considered high-risk and are judiciously utilized. Here we explore the safety of using organs from donors on ECMO support prior to donation.
*Methods: Retrospective review of the Gift of Life database identified abdominal transplants using ECMO-supported donor organs from ’06-’20. These were further cross-referenced with the Organ Procurement and Transplantation Network database. Transplants were stratified by organ – liver, kidney, liver-kidney (SLK) and pancreas-kidney (SPK). Outcomes using ECMO organs were compared to non-ECMO organs via Cox regression modeling. ECMO donors were categorized by type: venoarterial (VA) and venovenous (VV). ECMO type was compared through additional regression analyses.
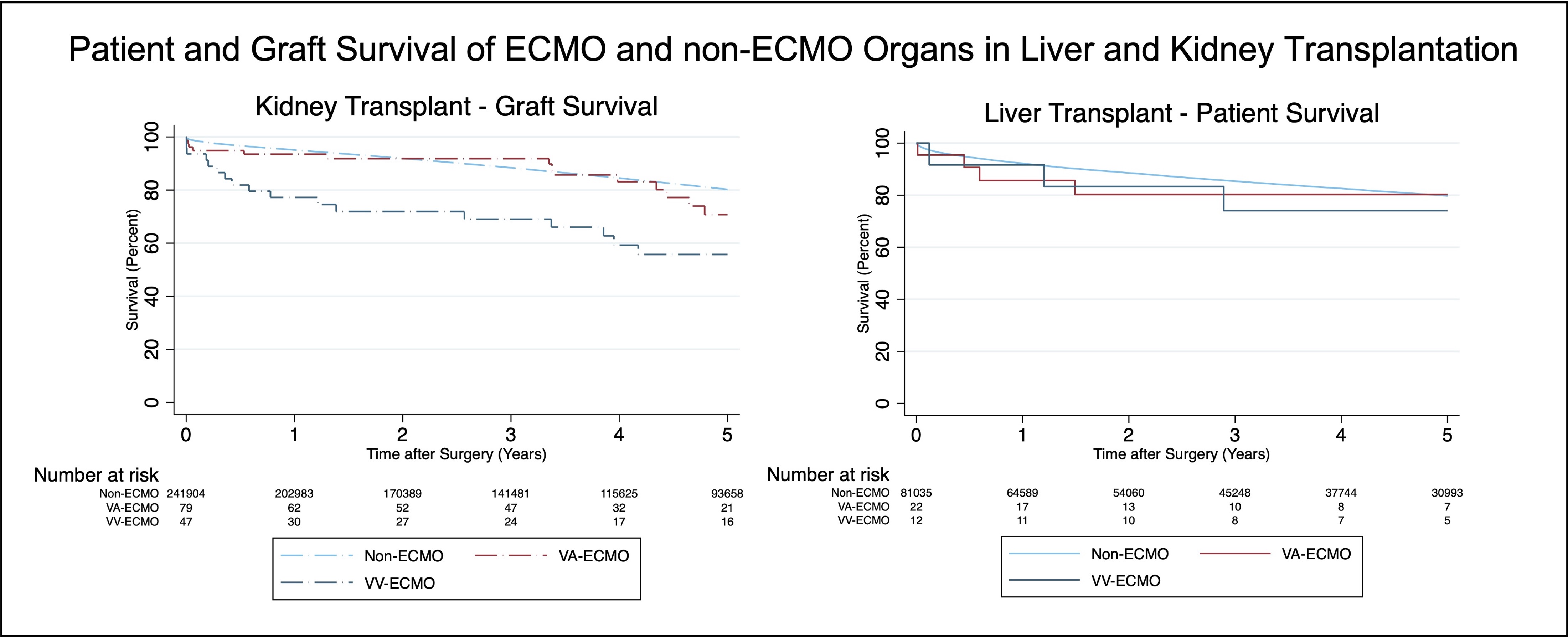
*Results: 168 transplants utilized donors supported by ECMO. This included 128 kidneys (80 VA, 48 VV), 34 livers (22 VA, 12 VV), 5 SLK (1 VA, 4 VV), and 1 VV SPK.In kidney transplants, 5-year graft survival was similar between non-ECMO and VA-ECMO donors on univariate (HR: 1.31, 95% CI: 0.8-2.2) and adjusted multivariable analyses accounting for donor and recipient factors (HR: 1.07, 95% CI: 0.6-1.8). VV-ECMO support was associated with worse graft survival (univariate HR: 3.00, 95% CI: 1.9-4.8; multivariable HR: 2.47, 95% CI: 1.5-4.0). Rates of kidney primary non-function, delayed graft function and follow-up creatinine were comparable between non-ECMO and VA-ECMO kidneys, but increased for VV-ECMO. Factors associated with graft failure for ECMO kidneys were acute inflammation on biopsy (HR: 8.71, 95% CI: 2.0-37.1), donor diabetes (HR: 4.49, CI: 1.4-14.5) and increased donor BMI (HR: 1.07, 95% CI: 1.0-1.1).In liver transplants, using ECMO and non-ECMO donors had similar 5-year patient survival upon univariate (HR: 1.29, 95% CI: 0.6-2.7) and multivariable regression (HR: 1.24, 95% CI: 0.6-2.8). Patient survival remained comparable between VA and VV-ECMO organs, as were rates of primary non-function.Remaining transplants included SLK and SPK. In SLK, patient and kidney graft survival at 5 years was 80%; in SPK, both kidney and pancreas grafts were functioning at 5 years.
*Conclusions: Kidney, liver and pancreas allografts procured from carefully selected donors supported by ECMO appear safe. Specific donor characteristics and further studies evaluating ECMO type are needed to understand poorer outcomes associated with VV ECMO.
To cite this abstract in AMA style:
Altshuler PJ, Shah AP, Glorioso JM, Maley WR, Frank AM, Ramirez CB, Dang H, West S, Hasz R, Bodzin AS. Assessing Safety of Using ECMO-Supported Donors in Abdominal Transplantation [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/assessing-safety-of-using-ecmo-supported-donors-in-abdominal-transplantation/. Accessed February 25, 2026.« Back to 2022 American Transplant Congress