Evaluation of Voriconazole Dosing for Preventing Aspergillus in Lung Transplant Recipients
1Pharmacy, Houston Methodist Hospital, Houston, TX, 2Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, TX, 3Medicine, Houston Methodist Hospital, Houston, TX
Meeting: 2022 American Transplant Congress
Abstract number: 1474
Keywords: Fungal infection, Infection, Lung infection, Lung transplantation
Topic: Clinical Science » Lung » 64 - Lung: All Topics
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: We sought to evaluate the efficacy of voriconazole (VCZ) 200 mg twice a day (BID) for 3 months post lung transplant (LT) in preventing Aspergillus (ASP) infection.
*Methods: A single-center, retrospective review was conducted on LT recipients who were initiated on VCZ 200 mg BID for 3 months between February 2016 – September 2020. Patients were excluded if transplanted for cystic fibrosis, received an azole other than VCZ, received an alternative VCZ dose, had prophylaxis started >14 days post-transplant, or death occurred within 30 days of transplant. The primary endpoint was defined as the incidence of (+) ASP cultures after receiving at least 5 days of VCZ to allow levels to approach steady state. Incidence of (+) ASP cultures was determined by the Kaplan Meier statistic. A univariable generalized linear model (GLM) analysis was conducted to compare characteristics of patients with (+) ASP cultures to patients with (-) cultures while on VCZ.
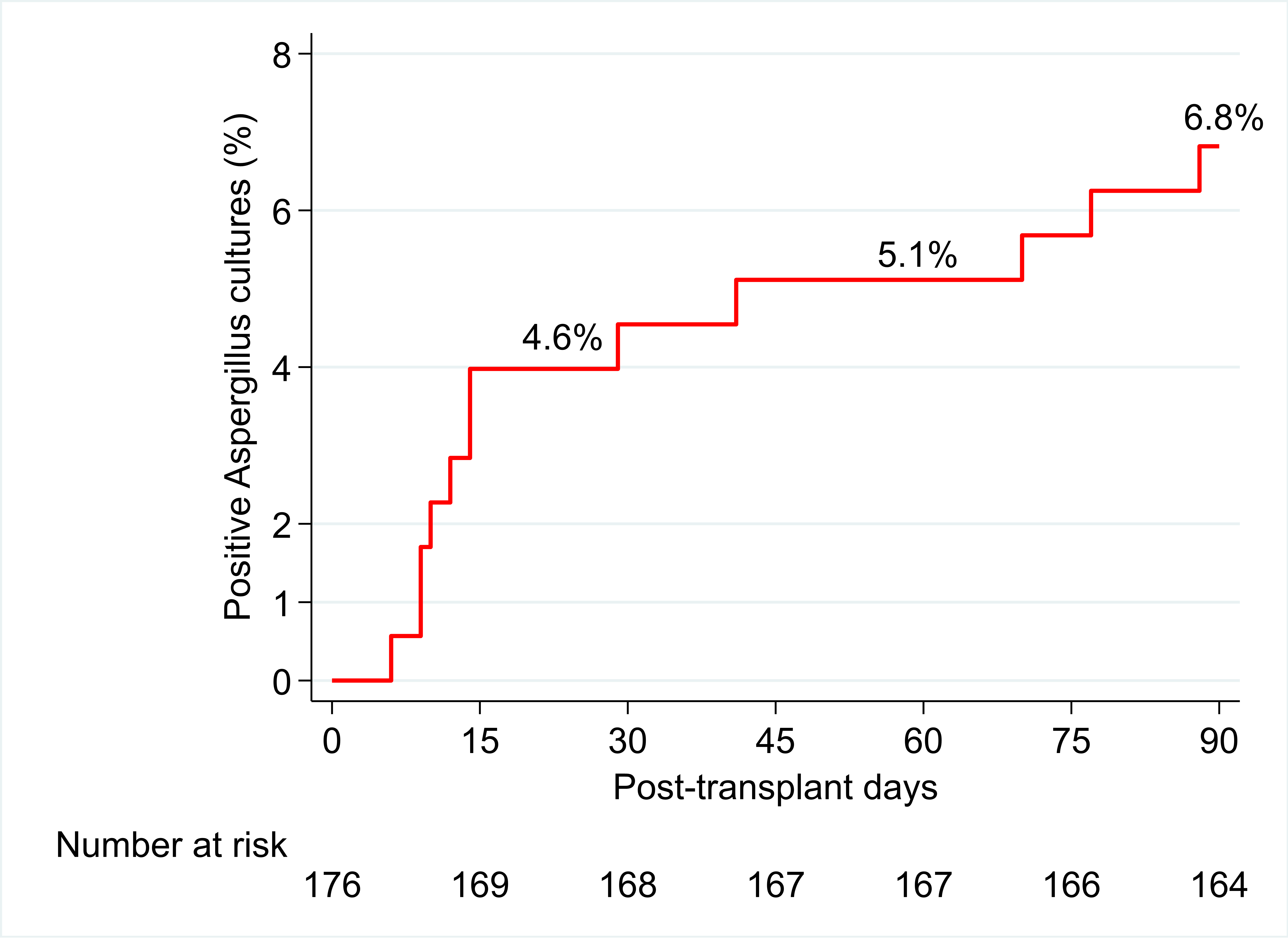
*Results: Three hundred twenty patients were transplanted during this time, 176 patients met inclusion criteria, and 12 patients (6.8%) experienced a breakthrough (+) ASP culture. Of patients who developed a (+) culture, 2 (17%) had a proven invasive infection and 7 (58%) had a probable invasive infection based off the IDSA consensus definitions for invasive fungal disease. Three (25%) patients had a (+) culture without any clinical features of infection and were classified as colonized with ASP. Patients with restrictive lung disease (diagnosis group D) had a higher risk of developing a (+) ASP culture than those with other indications for transplantation, p=0.03. No other statistical differences were noted between the cases versus controls when evaluating baseline characteristics and univariable GLM analysis.
*Conclusions: Patients who received 200 mg BID of VCZ for 3 months resulted in a low percentage of breakthrough (+) ASP cultures. Additionally, patients with restrictive lung disease were considered more likely to develop an ASP infection while receiving VCZ prophylaxis.
To cite this abstract in AMA style:
Chaplain A, Pierce BJ, Pham C, Hutchins A, Musick WL, Nguyen DT, Graviss EA, Yau SW, Youssef JG, Goodarzi A, Huang HJ. Evaluation of Voriconazole Dosing for Preventing Aspergillus in Lung Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-voriconazole-dosing-for-preventing-aspergillus-in-lung-transplant-recipients/. Accessed February 8, 2026.« Back to 2022 American Transplant Congress