SARS-CoV-2 Antibody Response by mRNA Vaccine Platform in Incrementally Immunosuppressed Patients
J. Mitchell1, C. Connolly1, T. Chiang1, J. Alejo1, W. Werbel1, D. Segev1, A. Massie2
1Johns Hopkins University, Baltimore, MD, 2Johns Hopkins School of Medicine, Baltimore, MD
Meeting: 2022 American Transplant Congress
Abstract number: 976
Keywords: COVID-19, Immunosuppression, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) II
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: This study compares SARS-CoV-2 antibody responses between the two-dose mRNA-1273 and BNT162b2 vaccine series across groups of incrementally immunosuppressed patients.
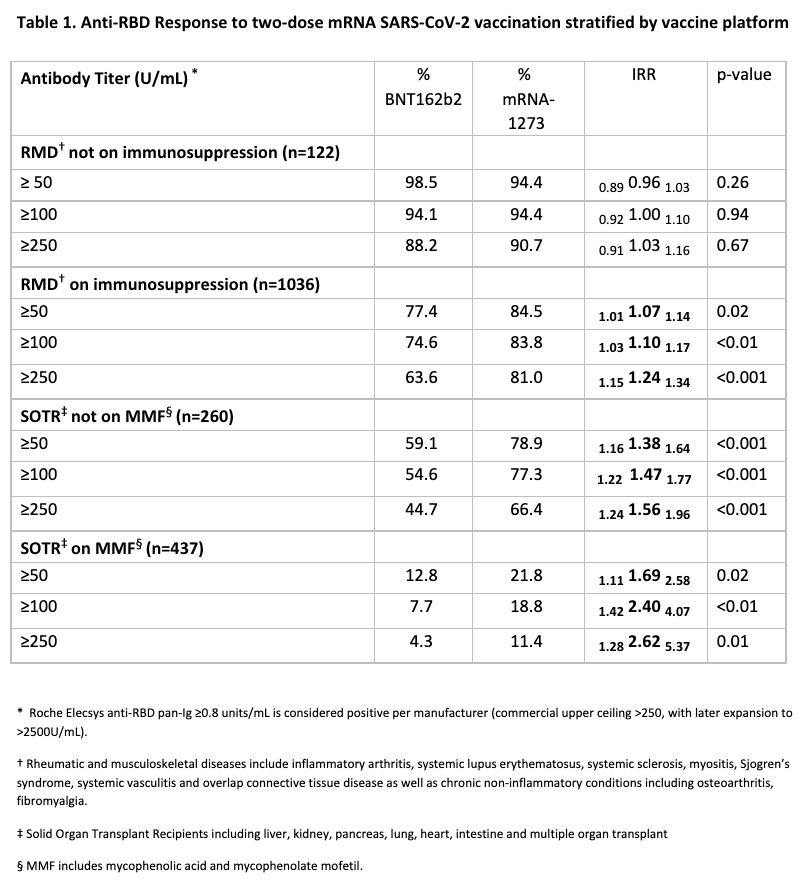
*Methods: Semiquantitative testing for antibodies against the receptor binding domain (RBD) of the SARS-CoV-2 spike protein was performed using the Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay (EIA), 15-45 days after the second vaccine dose for SARS-CoV-2 naïve patients with rheumatic and musculoskeletal disease (RMD), and solid organ transplant recipients (SOTRs) from an observational cohort. Anti-RBD titers were divided into categories of ≥50, ≥100 and ≥250 U/mL based on levels associated with plasma neutralizing capacity in COVID-19 convalescent patients. Participants were stratified by increasing intensity of immunosuppression: RMD not on immunosuppression, RMD on immunosuppression, SOTR not on mycophenolate (MMF), and SOTR on MMF. Response rates between mRNA-1273 and BNT162b2 recipients were compared using modified Poisson regression weighted for age, time since vaccination, and number of immunosuppressive medications. This analysis was repeated for several thresholds of positive response: 50, 100, and 250 U/mL.
*Results: Of 1868 participants, 55.8% of RMD and 52.7% of SOTRs received BNT162b2; the remainder received mRNA-1273. Demographics, diagnoses, and immunosuppressive regimens were similar across vaccine groups. Among RMD participants not on immunosuppression, the chance of anti-RBD ≥250U/ml was comparable among BNT162b2 and mRNA-1273 recipients (IRR= 0.91 1.03 1.16 p= 0.67). mRNA-1273 recipients had a higher chance than BNT162b2 recipients to achieve anti-RBD ≥250U/ml among RMD participants on immunosuppression (IRR = 1.15 1.24 1.34, p<0.001); SOTRs not on MMF (IRR = 1.24 1.56 1.96, p <0.001); and SOTRs on MMF (IRR= 1.28 2.62 5.37, p= 0.01). Similar trends were observed with titer cutoffs of ≥100 and ≥50 U/mL (Table 1).
*Conclusions: The two-dose mRNA-1273 vaccine series was more likely to induce stronger humoral immunogenicity compared to BNT162b2 in immunosuppressed patients; this effect was more pronounced with greater immunosuppression. These findings suggest importance in the choice of mRNA vaccine platform in optimizing immune responses to SARS-CoV-2 vaccination and can help inform vaccination strategies for booster doses in high-risk, immunosuppressed populations.
To cite this abstract in AMA style:
Mitchell J, Connolly C, Chiang T, Alejo J, Werbel W, Segev D, Massie A. SARS-CoV-2 Antibody Response by mRNA Vaccine Platform in Incrementally Immunosuppressed Patients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/sars-cov-2-antibody-response-by-mrna-vaccine-platform-in-incrementally-immunosuppressed-patients/. Accessed February 17, 2026.« Back to 2022 American Transplant Congress