An Analysis of Serological Response and Infection Outcomes Following Oxford Astra-Zeneca (azd1222) and Pfizer-Biontech (mRNA BNT162b2) SARS-CoV-2 Vaccines in Kidney and Pancreas Transplant Patients
A. Asderakis1, U. Khalid1, G. Koimtzis1, M. Ponsford2, L. Szabó1, L. Grant2, S. Jolles2, I. Humphreys3
1Cardiff Transplant Unit, University Hospital of Wales, Cardiff, United Kingdom, 2Immunodeficiency Centre for Wales, University Hospital of Wales, Cardiff, United Kingdom, 3Division of Infection and Immunity, Cardiff University, Cardiff, United Kingdom
Meeting: 2022 American Transplant Congress
Abstract number: 973
Keywords: Antibodies, COVID-19, Infection, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) II
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: SARS-CoV-2 is associated with high mortality among transplant recipients. This study aims to compare the humoral responses between the Oxford-Astra-Zeneca(AZ) and BNT162b2(Pfizer-BioNTech) vaccines in transplant recipients
*Methods: We recruited 920 kidney and SPK transplant patients receiving at least one dose of SARS-CoV-2 vaccine excluding patients with virus pre-exposure. Serological status was determined using the COVID-SeroKlir ELISA (Kantaro-EKF). Patients with corrected antibody level less than 0.7AU/mL were considered seronegative.
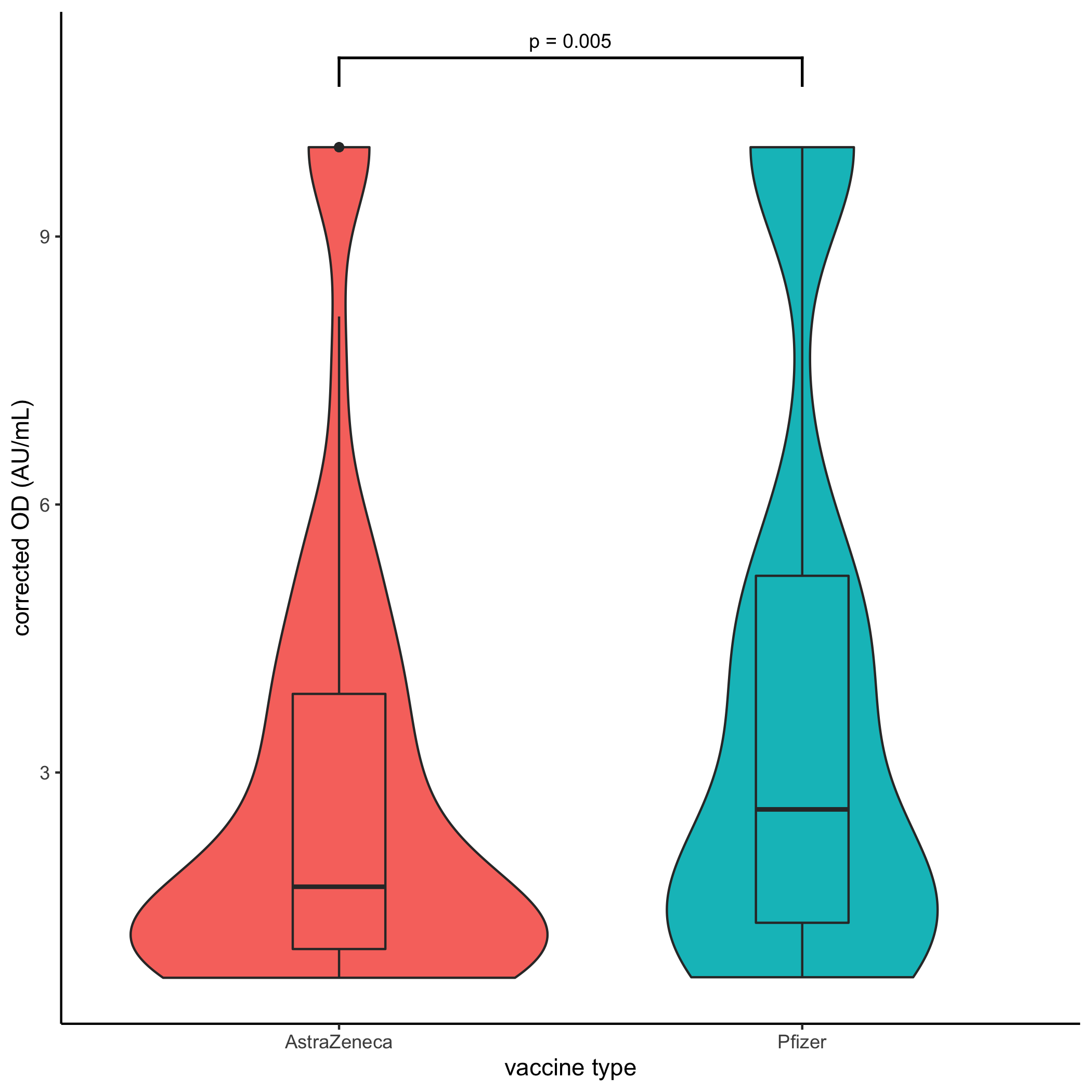
*Results: 495 AZ and 141 Pfizer patients had a sample post-first and 593 post-second dose (346 AZ vs 247 Pfizer) analysed. Following the 1st dose 25.7% of patients seroconverted (26.6% AZ and 22.8% Pfizer). Post-second dose 42.8% of AZ patients seroconverted (148/346) compared to 52.6% of Pfizer (130/247, p=0.02, HR 1.48, CI 1.07-2.06). When negative responders were excluded, Pfizer patients were shown to have a significantly higher response than AZ patients (median 2.6 vs 1.78AU/mL, Mann-Whitney p=0.005),
still lower than the one observed in general population. Patients on mycophenolate had a reduced seroconversion rate (42.2% vs 61.4%, p=0.001, HR 2.17) and reduced antibody levels (0.47 vs. 1.22 AU/mL, p=0.001) and this effect was dose dependent (p=0.05). Prednisolone reduced the seroconversion rate from 58.2% to 43.6% (p=0.03,HR 1.8) among Pfizer but not AZ recipients. This result was internally validated in two time points. Regression analysis has shown that antibody levels were reduced by older age (p=0.002), mycophenolate (p=0.001), AZ vaccine (vs Pfizer) (p=0.001) and male gender (p=0.02). There was no difference on infection rate post 2nd dose among the two vaccines but 14/15 serious post-vaccine infections leading to admission occurred to patients who did not seroconvert.
*Conclusions: Both seroconversion and antibody levels are lower following AZ compared to Pfizer vaccinated transplant patients following two vaccine doses. Mycophenolate, older age, male gender are also factors affecting the antibody response. Serious post vaccine infections are limited to patients without antibody response. Transplant patients remain at serious risk of SARS-CoV-2 infection.
To cite this abstract in AMA style:
Asderakis A, Khalid U, Koimtzis G, Ponsford M, Szabó L, Grant L, Jolles S, Humphreys I. An Analysis of Serological Response and Infection Outcomes Following Oxford Astra-Zeneca (azd1222) and Pfizer-Biontech (mRNA BNT162b2) SARS-CoV-2 Vaccines in Kidney and Pancreas Transplant Patients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/an-analysis-of-serological-response-and-infection-outcomes-following-oxford-astra-zeneca-azd1222-and-pfizer-biontech-mrna-bnt162b2-sars-cov-2-vaccines-in-kidney-and-pancreas-transplant-patients/. Accessed January 23, 2026.« Back to 2022 American Transplant Congress