Tacrolimus Metabolizer Heterogeneity within Caucasian Kidney Transplant (KT) Recipients
1Intermountain Medical Center, Murray, UT, 2CareDX, South San Francisco, CA
Meeting: 2022 American Transplant Congress
Abstract number: 1022
Keywords: Gene expression, Kidney, Pharmacokinetics
Topic: Clinical Science » Pharmacy » 29 - Non-Organ Specific: Pharmacokinetics / Pharmacogenomics / Drug interactions
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Weight-based tacrolimus (T) dosing is the standard in post-transplant KT. African-Americans and Caucasians differ in their metabolizer phenotype for T. However, two morphometrically similar individuals of the same race may have significant differences in T trough levels at the same dose. We integrated pharmacogenomics into clinical practice; hypothesizing that T variability in KT patients is guided by genomic profile and not by race in our predominantly Caucasian transplant population.
*Methods: Prospective single center study utilizing a proprietary pharmacogenetic gene panel (RxMatch) obtained by cheek swab. The panel reports on CYP2C19, CYP2C9, CYP2D6, CYP3A4, CYP3A5, and CYP4F2 expression. For CYP3A4 activity, patients were classified as intermediate metabolizers. CYP3A5 activity were classified as poor metabolizers, and intermediate metabolizers. T dose was determined by weight per protocol.
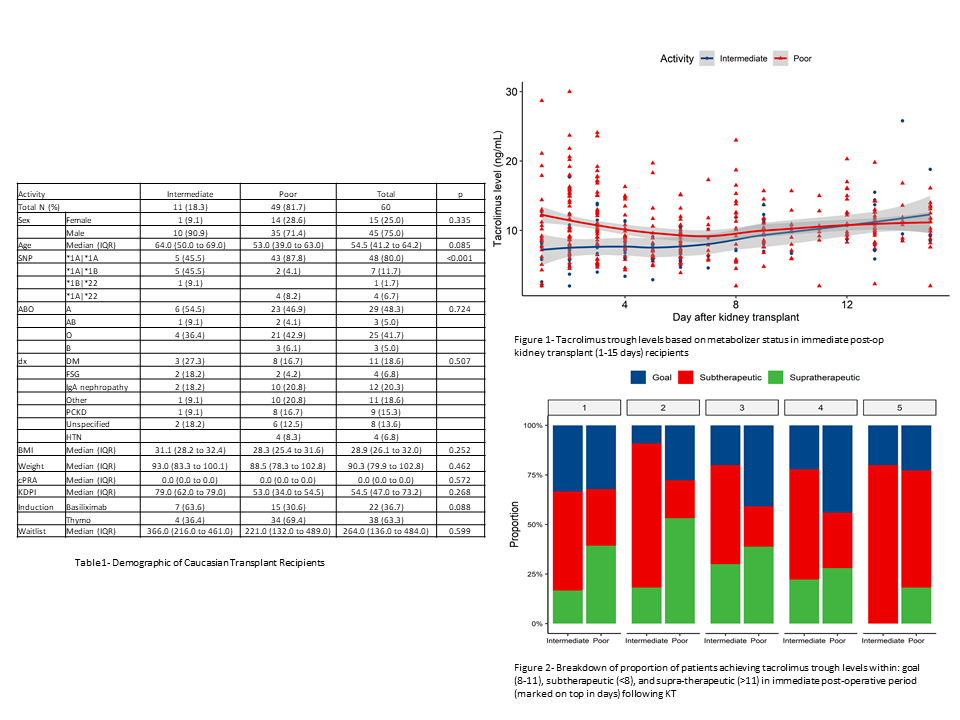
*Results: A total of 60 Caucasian pts (Jun ’17-Oct ’19) underwent RxMatch assessment. 45 (75%) patients were males and median age was 54.5 years (Table 1). Normal activity and intermediate metabolizers were identified in 55 (91.6%) and 5 (8.3%) patients, respectively. Concerning CYP3A5 activity, 49 (81.6%) and 11 (18.3%) patients were classified as poor metabolizers and as intermediate metabolizers, respectively. Patients with poor metabolizer status were more likely to develop supra therapeutic levels in the initial post-operative period until day 8, whereas intermediate metabolizers maintained subtherapeutic levels until post-op day 8 (Figure 1). At post KT day 2, a higher proportion of poor metabolizers achieved tacrolimus goal compared to intermediate metabolizers (27% vs 9%, p=0.002) (Figure 2). During the study period, 10 patients underwent ‘for cause’ biopsy. T-cell mediated rejection was identified in 1/1 and 5/9 patients at a median of three months in the intermediate and poor metabolizer, respectively.
*Conclusions: We demonstrate significant pharmacogenetic heterogeneity, in tacrolimus metabolizer status within the Caucasian population. We demonstrate the potential use case of pharmacogenetics as a lead measure guiding precise dosing of tacrolimus. Dose adjustment based solely on a weight based regimen, can lead to delayed attainment of tacrolimus therapeutic levels. Incorporation of pharmacogenomic data to guide decision regarding tacrolimus dosing early may have potential downstream cost savings, with goal ranges achieved early on rather than later, and hence requiring fewer blood draws.
To cite this abstract in AMA style:
Sanchez-Garcia J, Oliver N, Morris D, Raza A, Lindberg L, LeCorhick S, Srinivas T, Anand S. Tacrolimus Metabolizer Heterogeneity within Caucasian Kidney Transplant (KT) Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/tacrolimus-metabolizer-heterogeneity-within-caucasian-kidney-transplant-kt-recipients/. Accessed February 28, 2026.« Back to 2022 American Transplant Congress