Pharmacogenomic Dosing in Kidney Transplant Patients Converted from Tacrolimus Immediate Release to Tacrolimus Extended Release (Envarsus XR®)
1Department of Pharmacy, Yale New Haven Hospital, New Haven, CT, 2Department of Pharmacy, Hospital of Central Connecticut, New Britain, CT, 3Section of Nephrology, Yale School of Medicine, New Haven, CT
Meeting: 2022 American Transplant Congress
Abstract number: 1021
Keywords: Genomics, Immunosuppression, Kidney transplantation
Topic: Clinical Science » Pharmacy » 29 - Non-Organ Specific: Pharmacokinetics / Pharmacogenomics / Drug interactions
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: When converting from tacrolimus (tac) immediate release (tacIR) to tac extended release (Envarsus XR; tacXR), the package insert (PI) recommends 80% of the tacIR total daily dose (TDD). Precision tac dosing using pharmacogenomic (pgx) testing and other clinical data can provide insight to appropriate starting doses. Limited data exists on the use of pgx to predict tacXR dosing. The purpose of this study was to compare kidney transplant recipient (KTR)’s stable dose of tacXR to their predicted starting dose of tacIR based on the Clinical Pharmacogenetics Implementation Consortium (CPIC) and a validated tac dosing algorithm recommendations.
*Methods: This was a retrospective chart review of adult KTRs transplanted between 4/2019 and 1/2020. Patients were included if they completed pgx testing and converted to tacXR post-transplant with at least one therapeutic trough. Two recommended tacIR starting doses were calculated: CPIC and a validated algorithm. The primary outcome was the % of CPIC and algorithm predicted tacIR TDD that resulted in a stable tacXR dose defined by first therapeutic trough (5-8 ng/mL) taken at least 5 days after conversion. Secondary outcomes included comparison of the % of predicted tacIR TDD that resulted in a stable tacXR dose in CYP3A5 expressers and non-expressers.
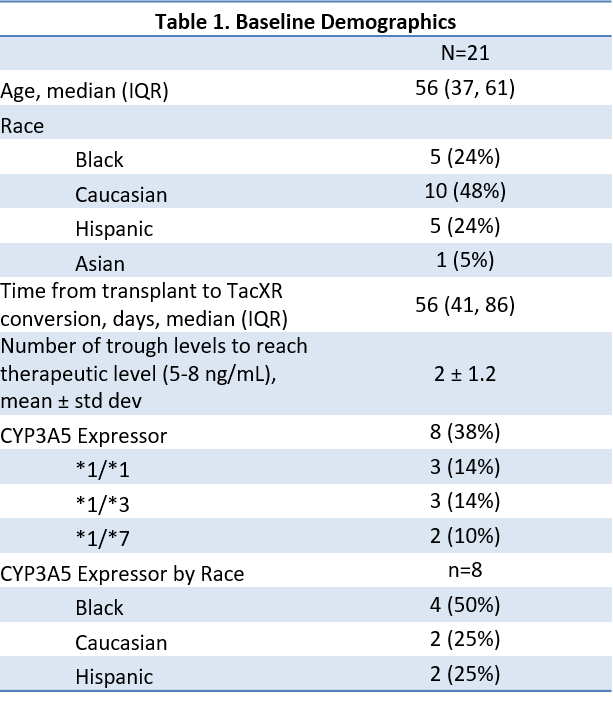
*Results: 21 patients were included. Baseline demographics are presented in Table 1. The median stable tacXR dose was 73% of the CPIC recommended tacIR starting dose and 87% of the algorithm recommended tacIR starting dose. There was no significant difference in % of recommended dose between CYP3A5 expressors and non-expressors, however there was greater variability among non-expressers between CPIC and algorithm recommendations.
*Conclusions: The PI for tacXR recommends converting from tacIR at 80% of the TDD. After conversion, the stable dose of tacXR was predicted accurately by both CPIC and algorithm recommendations, 73-87% of tacIR TDD. Future studies are needed to validate the use of pgx for tacXR.
To cite this abstract in AMA style:
Belfield KD, Armstrong KJ, Malhotra D, Girone GF. Pharmacogenomic Dosing in Kidney Transplant Patients Converted from Tacrolimus Immediate Release to Tacrolimus Extended Release (Envarsus XR®) [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/pharmacogenomic-dosing-in-kidney-transplant-patients-converted-from-tacrolimus-immediate-release-to-tacrolimus-extended-release-envarsus-xr/. Accessed January 13, 2026.« Back to 2022 American Transplant Congress