Impact of Glecaprevir/Pibrentasvir on Tacrolimus Concentration in Solid Organ Transplant Recipients with or without Triazole Antifungal Therapy
D. Abraham1, A. Feist2, N. Law1, S. Aslam1, V. Nguyen1
1University of California San Diego Health, San Diego, CA, 2University of California San Diego, San Diego, CA
Meeting: 2022 American Transplant Congress
Abstract number: 1020
Keywords: Drug interaction, Hepatitis C, Immunosuppression, Viral therapy
Topic: Clinical Science » Pharmacy » 29 - Non-Organ Specific: Pharmacokinetics / Pharmacogenomics / Drug interactions
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Provide guidance on tacrolimus (TAC) dose adjustments during glecaprevir/pibrentasvir (GLE/PIB) initiation and discontinuation in patients on triazole antifungals by comparing changes in TAC concentration-dose ratio (C/D) with or without triazole antifungal therapy.
*Methods: A single-center retrospective study of adults transplanted between January 1st, 2017 and August 1st, 2021 who received concomitant TAC and GLE/PIB for 12 weeks was conducted. TAC C/D was compared between groups based on degree of CYP3A inhibition by triazole used during GLE/PIB therapy: strong (posaconazole or voriconazole), moderate (fluconazole), and no triazole. Kruskal-Wallis and Wilcoxon Signed Ranks tests were used to assess outcomes.
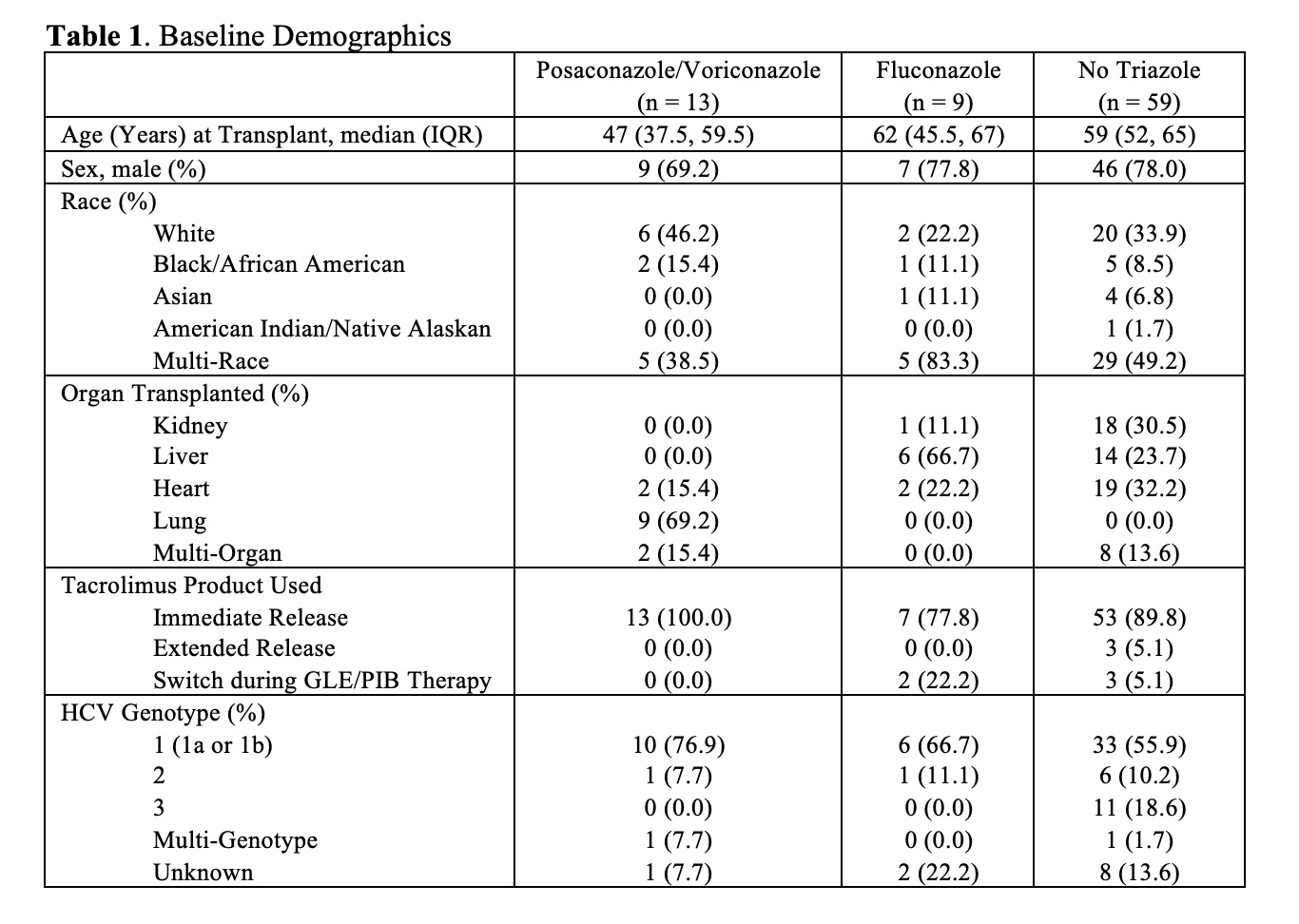
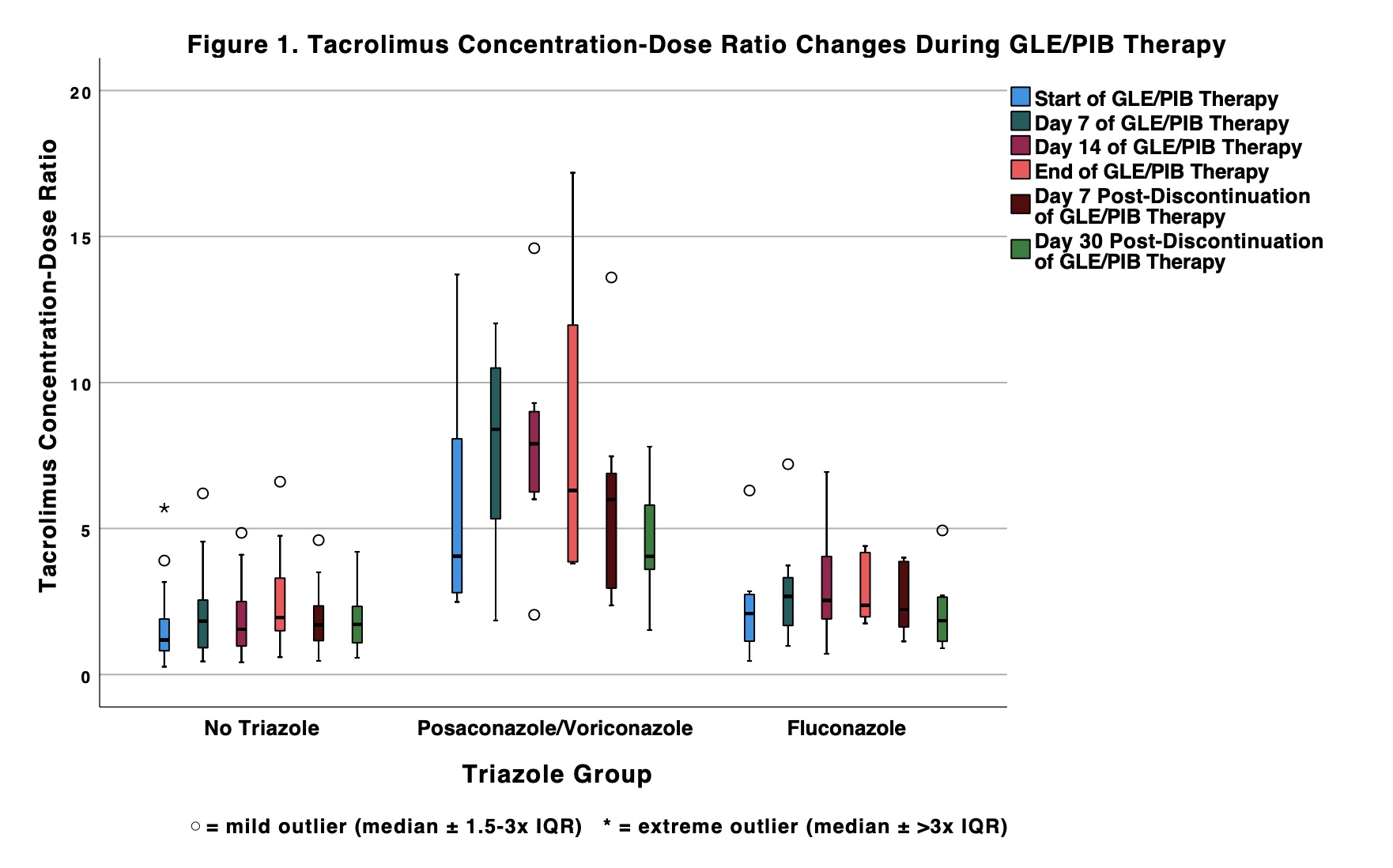
*Results: A total of 81 patients who received concomitant tacrolimus and GLE/PIB were included: 13 (16.0%) also received posaconazole or voriconazole, 9 (11.1%) fluconazole, and 59 (72.8%) no triazole. TAC C/D differed significantly at all time points in the no triazole group (p < 0.001), from end of GLE/PIB therapy to 30 days post-discontinuation in the posaconazole/voriconazole group (p = 0.023), and from baseline to day 7 and end of therapy to 7 days post-discontinuation in the fluconazole group (p = 0.011 and 0.012, respectively). Percent change in TAC C/D from baseline to day 14 was numerically higher in the posaconazole/voriconazole group albeit not statistically significant (64.0 vs. 20.7 [fluconazole] vs. 38.8 [no triazole], p = 0.737). Percent change in TAC C/D at GLE/PIB initiation and discontinuation and time to hepatitis C virus clearance did not differ significantly among groups (Table 2).
*Conclusions: Increased TAC monitoring may be required at initiation and discontinuation of GLE/PIB, regardless of triazole use. Hepatitis C clearance did not change significantly with the addition of a triazole. Further research in a larger cohort is needed to determine impact of posaconazole and voriconazole on this interaction.
To cite this abstract in AMA style:
Abraham D, Feist A, Law N, Aslam S, Nguyen V. Impact of Glecaprevir/Pibrentasvir on Tacrolimus Concentration in Solid Organ Transplant Recipients with or without Triazole Antifungal Therapy [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-glecaprevir-pibrentasvir-on-tacrolimus-concentration-in-solid-organ-transplant-recipients-with-or-without-triazole-antifungal-therapy/. Accessed February 16, 2026.« Back to 2022 American Transplant Congress