Experience with Covid-19 Infection and Vaccination in Combined Kidney/HSCT Recipients
M. Gibson1, D. Belshe1, G. Gregory1, S. Tanna1, M. Ison1, S. Ildstad2, J. R. Leventhal1
1Northwestern University, Chicago, IL, 2University of Louisville, Louisville, KY
Meeting: 2022 American Transplant Congress
Abstract number: 985
Keywords: Kidney transplantation, Outcome, Stem cells, Tolerance
Topic: Clinical Science » Infection Disease » 25 - Kidney Infectious Non-Polyoma & Non-Viral Hepatitis
Session Information
Session Name: Kidney Infectious Non-Polyoma & Non-Viral Hepatitis
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Hall C
*Purpose: Kidney transplant(KTx) recipients are at increased risk of COVID-19 related complications. KTx pts who have undergone therapy to induce tolerance are a unique cohort regarding SARS-CoV-2 susceptibility, clinical course following infection, and vaccine response. We have reported results of a phase 2 trial to induce tolerance through establishment of durable donor whole blood and T-cell chimerism using FCR001, an investigational cell therapy. Pts received nonmyeloablative conditioning, LD KTx, FCR001 infusion and weaning of IS as previously described (Leventhal et al STM 2012). Full withdrawal of immunosuppression (IS) was possible in 26 of 37 pts, with a low (5.5%) risk of graft versus host disease (GvHD). We evaluated the impact of COVID-19 infection and vaccination response in this cohort.
*Methods: A chart review of pts was conducted; variables of interest included demographic data, clinical course, and virologic assays.
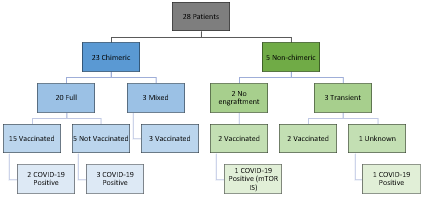
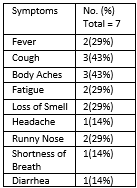
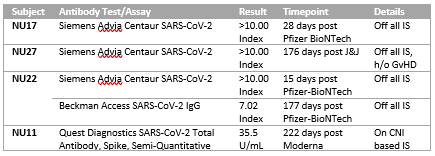
*Results: We identified 28 evaluable pts (Figure 1), 7 of which tested Covid positive. No infected pts were hospitalized; there was no evidence of renal function impairment, mean eGFR pre-infection was 57.83 mL/min/1.73 m2 and post-infection was 59.25 mL/min/1.73 m2. No change in chimerism was seen with infection. Three infected pts received casirivimab/imdevimab. Reported symptoms during COVID-19 infection were mild (Table 1). 22 pts have been vaccinated (16 Pfizer-BioNtech, 3 Johnson&Johnson(J&J), and 3 Moderna). 4 pts had serology performed (Table 2). Vaccination was well tolerated with no loss of chimerism.
*Conclusions: No KTx/FCR001 pts with COVID-19 developed a severe disease type. Infection in pts off IS was not associated with loss of chimerism. SARS-CoV-2 vaccination resulted in strong humoral responses and did not lead to loss of chimerism or allograft dysfunction.
To cite this abstract in AMA style:
Gibson M, Belshe D, Gregory G, Tanna S, Ison M, Ildstad S, Leventhal JR. Experience with Covid-19 Infection and Vaccination in Combined Kidney/HSCT Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/experience-with-covid-19-infection-and-vaccination-in-combined-kidney-hsct-recipients/. Accessed February 11, 2026.« Back to 2022 American Transplant Congress