Intermediate Outcomes Following Early Inflammatory Changes on Surveillance Biopsies
1Starzl Transplant Institute, University of Pittsburgh Medical Center, Pittsburgh, PA, 2Department of Renal-electrolyte, University of Pittsburgh Medical Center, Pittsburgh, PA
Meeting: 2021 American Transplant Congress
Abstract number: 1067
Keywords: Biopsy, Kidney transplantation, Outcome, Rejection
Topic: Clinical Science » Kidney » Kidney Complications: Immune Mediated Late Graft Failure
Session Information
Session Name: Kidney Complications: Immune Mediated Late Graft Failure
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: The implications on early inflammatory changes on surveillance biopsies on intermediate and long-term outcomes is unclear.
*Methods: Of the 1000 kidney transplant (live donor and deceased donor) recipients at our center between Jan 2013 through Dec 2017, we prospectively followed a cohort of 518 recipients who had 3 month surveillance biopsies. These were divided into Group 1 – no major abnormality (NMA; i0t0 and i>0;t0; n=278); Group 2 – Borderline Changes (BL-R; i≥1;t≥1 but not meeting criteria for Banff IA; n=161) and Group 3 – Subclinical T cell mediated rejection (SCR; i2t2 and higher; n=79). We excluded patients who could not undergo 3 m surveillance biopsies and those who had clinical rejections prior to index biopsy. BL-R was treated in a minority of cases with one dose of 250 mg of methylprednisolone, while SCR was treated with methylprednisolone 250 mg iv x 3. Baseline demographics between the groups including recipient age, race, sex, prior transplant and sensitization was similar between the groups. Majority of the patients received thymoglobulin induction (>95%) and maintenance IS with tac and MMF. Median period of follow up was 4.5 years (IQR 3.5-7.8). We studied the composite endpoint of subsequent clinical rejections and death censored graft loss (DCGL) in all groups and also the hard endpoint of DCGL alone.
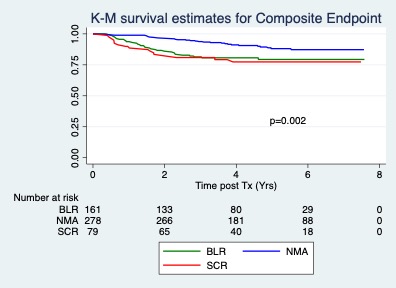
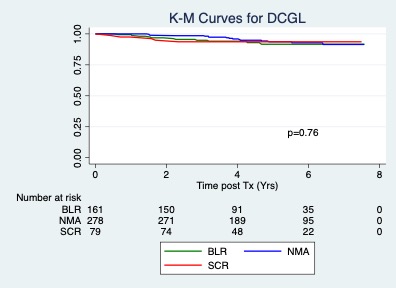
*Results: Figure 1. Figure 2.
*Conclusions: 1. The hazard ratio for reaching the composite endpoint of clinical rejection or DCGL increased in the BL-R and SCR groups compared to NMA (HR 2.3; p=0.001; 95% CI 1.4-3.7). 2. The hard endpoint of DCGL was not different among the groups in the intermediate term. 3. Long term follow up will be helpful to detect any long term implications of early inflammatory changes on surveillance biopsies
To cite this abstract in AMA style:
Melgarejo I, Viswanathan V, Sharma A, Sood P, Puttarajappa C, Shah N, Molinari M, Tevar A, Wu C, Hariharan S, Mehta R. Intermediate Outcomes Following Early Inflammatory Changes on Surveillance Biopsies [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/intermediate-outcomes-following-early-inflammatory-changes-on-surveillance-biopsies/. Accessed February 8, 2026.« Back to 2021 American Transplant Congress