Clazakizumab for the Treatment of Chronic Active Antibody-Mediated Rejection in Kidney Transplant Recipients: Phase 3 IMAGINE Study Design
1University of Manitoba, Winnipeg, MB, Canada, 2Medical University of Vienna, Vienna, Austria, 3University of Sydney, Sydney, Australia, 4University of Toronto, Toronto, ON, Canada, 5University of Nebraska Medical Center, Omaha, NE, 6Leiden University Medical Center, Leiden, Netherlands, 7CSL Behring, King of Prussia, PA, 8University of Wisconsin, Madison, WI
Meeting: 2021 American Transplant Congress
Abstract number: 1047
Keywords: Graft failure, Kidney/liver transplantation
Topic: Clinical Science » Kidney » Kidney Chronic Antibody Mediated Rejection
Session Information
Session Name: Kidney Chronic Antibody Mediated Rejection
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Chronic active antibody-mediated rejection (CAMR) is a major cause of allograft loss with no approved drugs for its treatment. Currently, off-label regimens recommended by experts based on small clinical trials are used, reflecting the high unmet medical need for effective therapies based on well-controlled trials. Clazakizumab (CLAZA) is a high-affinity, humanized monoclonal antibody that binds IL-6 and blocks inflammation and antibody production. Small Phase 2 studies of CLAZA in kidney transplant recipients with CAMR suggest early modulation of donor-specific antibodies (DSA), stabilization of GFR, and a manageable safety profile. In CAMR, a progressive decline in kidney function is, by definition, on the pathway to graft loss. Here, we report the design of the Phase 3 IMAGINE study (NCT03744910) to evaluate the safety and efficacy of CLAZA for the treatment of CAMR.
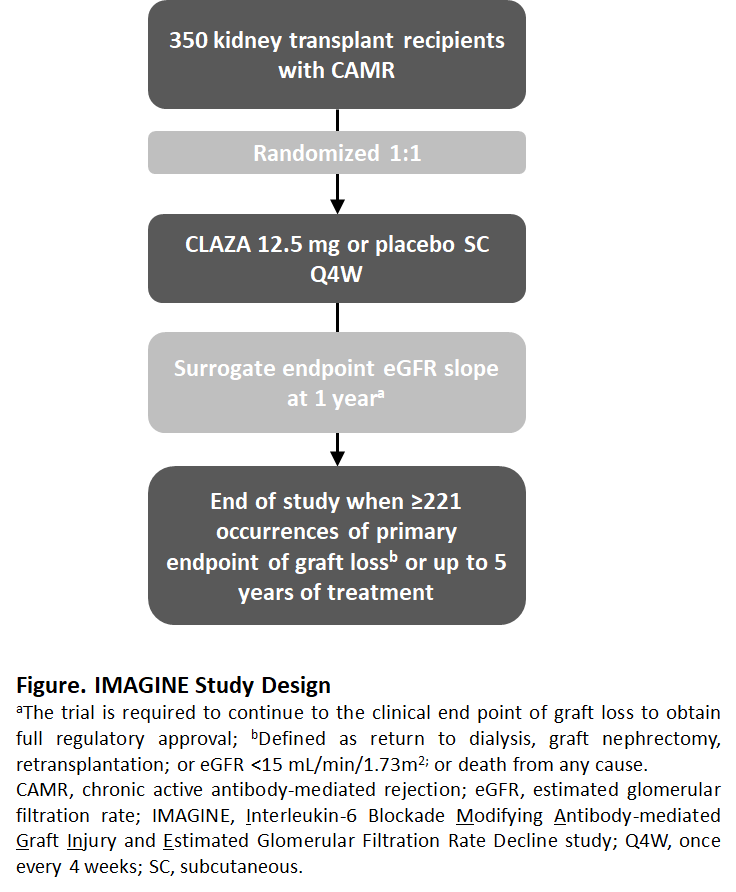
*Methods: IMAGINE is a multi-center, double-blind trial of 350 kidney transplant recipients with CAMR (Banff cg>0 with human leukocyte antigen DSA) randomized 1:1 to receive CLAZA (12.5 mg subcutaneous once every 4 weeks) or placebo (Fig). The event-driven trial design will follow pts for 5 years or until 221 occurrences of graft loss (primary endpoint); defined as return to dialysis, graft nephrectomy, retransplantation, eGFR <15 mL/min/1.73m2, or death from any cause. A surrogate for graft loss, eGFR slope at 1 year, will be assessed based on prior modelling validation1. Secondary endpoints will include measures of PK/PD and PROs.
*Results: Recruitment is ongoing across sites in N. America, Europe, Asia, and Australia.
*Conclusions: Currently, no treatment has proven effective in CAMR. This pivotal Phase 3 trial includes prognostic biomarker enrichment and uniquely incorporates a surrogate endpoint for graft loss, eGFR slope at 1 year, which may accelerate the approval of a novel therapy for patients at risk of allograft loss.
References
1. Irish W. et al. Transplantation. 2020; Epub ahead of print. doi: 10.1097/TP.0000000000003274.
To cite this abstract in AMA style:
Nickerson P, Böhmig G, Chadban S, Kumar D, Mannon RB, Gelder Tvan, Adler S, Chong E, Djamali A. Clazakizumab for the Treatment of Chronic Active Antibody-Mediated Rejection in Kidney Transplant Recipients: Phase 3 IMAGINE Study Design [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/clazakizumab-for-the-treatment-of-chronic-active-antibody-mediated-rejection-in-kidney-transplant-recipients-phase-3-imagine-study-design/. Accessed February 19, 2026.« Back to 2021 American Transplant Congress