Donor-Derived Tuberculosis in Lung Transplant Recipients, a Single Center Experience
M. Robbins, D. Kabbani, C. Cervera, C. O'Neil, R. Cooper, K. Halloran, S. Grocholski, K. Doucette
University of Alberta, Edmonton, AB, Canada
Meeting: 2021 American Transplant Congress
Abstract number: 760
Keywords: Bacterial infection, Donation, Infection, Lung
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Donor-derived (DD) tuberculosis (TB) in solid-organ transplantation (SOT) is rare with the largest review to date identifying only 25 cases of proven or probable DD-TB.
*Methods: We describe the largest case-series of DD-TB to date, including four cases among lung transplant recipients between 2015-2020 with a focus on identifying risk factors for transmission and characterizing clinical presentations.
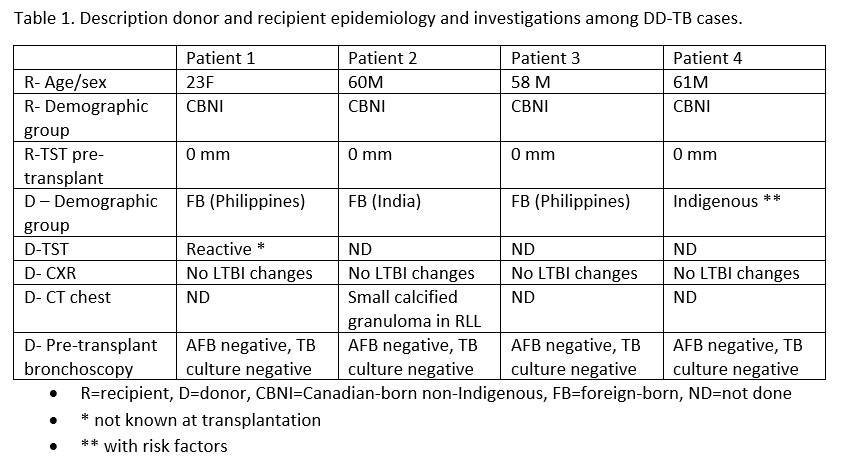
*Results: All affected recipients were Canadian-born non-Indigenous lung transplant recipients with negative tuberculin skin tests (TST) and without epidemiologic risks for TB infection. In terms of donors, 3/4 were foreign-born with the fourth being Canadian-born but with epidemiologic risks for TB including Indigenous status, homelessness and alcohol abuse. One donor had a prior reactive TST (unknown at donation) and one donor had prior imaging demonstrating a small calcified pulmonary granuloma. All cases presented within three months post-transplant (range 7-12 weeks) with fever and dyspnea in all recipients. Imaging revealed nodular opacities and mediastinal lymphadenopathy in all. One recipient was both smear and culture positive, while the other three recipients were smear negative and culture positive. Three presented with isolated pulmonary TB while the fourth was diagnosed with disseminated TB. Treatment in one case included isoniazid (INH), rifabutin (RFB), and pyrazinamide (PYR) induction followed by INH and RFB maintenance for a total of 12 months with clinical cure. The second case was treated with RFB, PYR, and moxifloxacin induction followed by RFB and moxifloxacin maintenance for a total of nine months with clinical cure. The third was treated with INH, RFP, ethambutol, and moxifloxacin induction followed by INH and RFP maintenance for a total of 9 months. The fourth case was initiated on INH, RFB, and moxifloxacin but died within one week of treatment initiation.
*Conclusions: In summary, DD-TB remains an uncommon complication following SOT. Our case series confirms donor TST reactivity, presence of lung granulomata, and epidemiologic risks including country of origin, Indigenous status, homelessness, and alcohol abuse are associated with increased risk of DD-TB. This suggests enhanced communication between jurisdictions with respect to prior LTBI testing and chest imaging may improve identification of donors with LTBI. However, some donors may still pose a risk of TB transmission, suggesting a possible role for enhanced screening or pre-emptive LTBI therapy in this group.
To cite this abstract in AMA style:
Robbins M, Kabbani D, Cervera C, O'Neil C, Cooper R, Halloran K, Grocholski S, Doucette K. Donor-Derived Tuberculosis in Lung Transplant Recipients, a Single Center Experience [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/donor-derived-tuberculosis-in-lung-transplant-recipients-a-single-center-experience/. Accessed January 28, 2026.« Back to 2021 American Transplant Congress