Current Practices for Management and Treatment of COVID-19 in Immunocompromised Adults: A Survey of Institutions
H. H. Nam1, S. Aslam2, O. Beaird3, G. Forrest4, M. Fung5, A. Limaye6, A. Multani3, J. Nelson7, R. Rakita6, L. Strasfeld8, J. Schaenman3
1Medicine, University of California - Irvine, Orange, CA, 2Division of Infectious Diseases and Global Public Health, University of California - San Diego, San Diego, CA, 3Medicine, University of California - Los Angeles, Los Angeles, CA, 4Medicine, Rush University, Chicago, IL, 5Medicine, University of California - San Francisco, San Francisco, CA, 6Medicine, University of Washington, Seattle, WA, 7Medicine, Stanford University, Palo Alto, CA, 8Medicine, Oregon Health and Science University, Portland, OR
Meeting: 2021 American Transplant Congress
Abstract number: 753
Keywords: Donors, unrelated, Infection, Viral therapy
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: The optimal testing strategy for solid organ transplantation (SOT) donor and recipient evaluation, as well as treatment for COVID-19 is unknown. We assessed the management strategy of COVID-19 within the West Coast Transplant Infectious Disease group.
*Methods: A survey assessing strategies for COVID-19 management was sent to 11 Transplant Infectious Diseases providers from 8 centers.
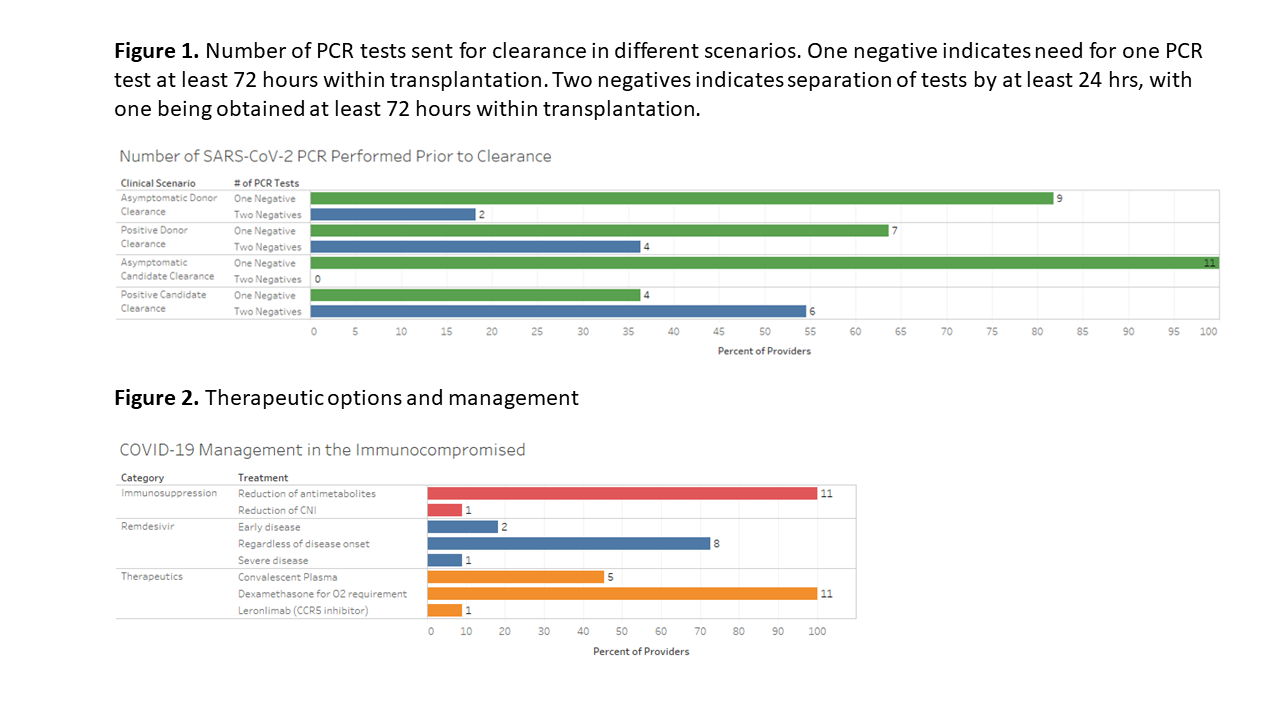
*Results: For both living and deceased donor clearance, 81.8% (n=9) providers utilize one negative PCR within 72 hours of transplantation. However, when a living donor tests positive for SARS-COV-2, 36.4% (n=4) will require two negative PCR tests >24 hours apart for donor clearance, with 90.9% (n=10) requiring at least a 28-day wait period prior to retesting. Amongst providers caring for lung transplant recipients, 77.7% (7/9) utilized negative PCR from donor BAL as part of pre-transplant evaluation. For transplant candidates, all providers required both the absence of COVID-19 symptoms with only one negative PCR test. In candidates that tested positive for SARS-COV-2, 54.5% (n=6) required at least two negative PCRs prior to transplantation. For positive candidates, 63.6% (n=7) considered re-testing for PCR negativity at 20 days. When a transplant recipient tested positive for SARS-CoV-2, all providers would reduce antimetabolites and utilized dexamethasone for patients requiring oxygen therapy. Remdesivir was prescribed by all providers with variability in the timing of administration.
*Conclusions: Management and treatment of COVID-19 for SOT donors, candidates, and recipients was heterogeneous. While all providers require at least one negative COVID-19 test as both donor and recipient evaluation prior to transplantation, the number of negative tests sent varied amongst providers, geographical region, and clinical scenario. The significant diversity of COVID-19 management strategies for immunocompromised adults seen in this study highlights the further need for studies defining the optimal management of COVID-19.
To cite this abstract in AMA style:
Nam HH, Aslam S, Beaird O, Forrest G, Fung M, Limaye A, Multani A, Nelson J, Rakita R, Strasfeld L, Schaenman J. Current Practices for Management and Treatment of COVID-19 in Immunocompromised Adults: A Survey of Institutions [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/current-practices-for-management-and-treatment-of-covid-19-in-immunocompromised-adults-a-survey-of-institutions/. Accessed March 6, 2026.« Back to 2021 American Transplant Congress