The Role of Pre-transplant Rectal Screening for Azole-resistant Candida Species in Liver Transplant Candidates
K. K. Patel1, M. M. Azar2, A. Koff3, K. Belfield4, D. R. Peaper5, J. Topal2, M. Malinis2
1Yale School of Medicine, New Haven, CT, 2Section of Infectious Diseases, Yale School of Medicine, New Haven, CT, 3Section of Infectious Diseases, UC Davis, Sacramento, CA, 4Department of Pharmacy, Yale New Haven Hospital, New Haven, CT, 5Department of Laboratory Medicine, Yale School of Medicine, New Haven, CT
Meeting: 2021 American Transplant Congress
Abstract number: 314
Keywords: Fungal infection, Liver transplantation, Post-operative complications, Screening
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: Disparities in Access and Machine Learning Outcomes in Solid Organ Transplantation
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 8, 2021
Session Time: 4:30pm-5:30pm
 Presentation Time: 4:55pm-5:00pm
Presentation Time: 4:55pm-5:00pm
Location: Virtual
*Purpose: Azole-resistant Candida (ARC) infections have become increasingly common among solid organ transplant recipients. Pre-transplant screening for ARC allows for targeted antifungal prophylaxis, which may reduce the incidence of post-transplant ARC infection. In this study, we describe a single-center experience of routine ARC screening in liver transplant (LT) candidates.
*Methods: We performed a retrospective chart review of patients who underwent LT at Yale-New Haven Hospital from April 2019 to November 2020. ARC (C. glabrata and C. krusei) screening was performed via a rectal swab prior to or at time of LT. Collected data included patient demographics, ARC screening, antifungal prophylaxis, risk factors associated with Candida infection (previously defined by AST ID COP guidelines), and Candida infection within 1 month of LT.
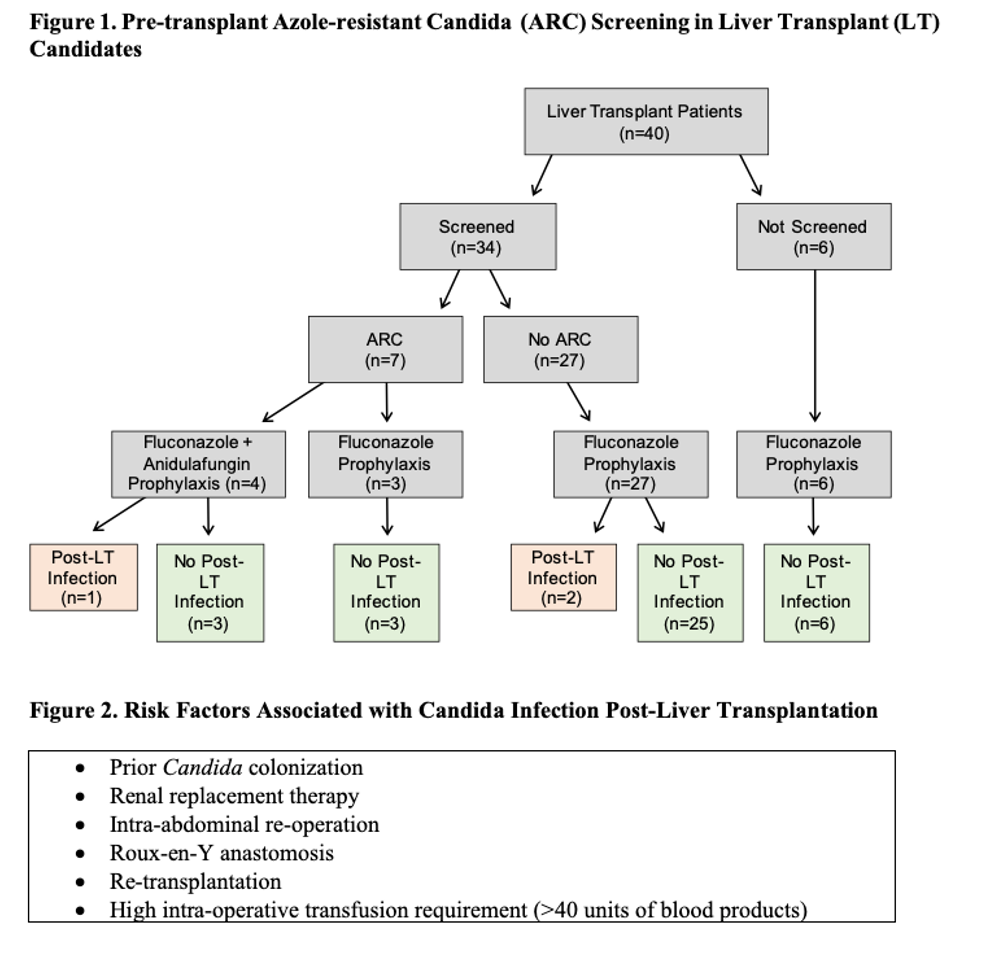
*Results: Forty patients underwent LT. The median age was 59 years (range 25-70), 27 (68%) were male, and 19 (48%) had a MELD ≥ 30 at time of LT. Of this cohort, 34 (85%) had ARC screening. ARC colonization was present in 7/34 (21%) patients: C. glabrata (n=4), C. krusei (n=2), and both species (n=1). All 7 patients received antifungal prophylaxis. Echinocandin prophylaxis was given in 4/7 (57%), of whom 1 developed confirmed ARC infection (C. krusei and C. glabrata fungemia). Three ARC colonized patients received fluconazole but none developed post-LT Candida infection. In non-ARC colonized patients on fluconazole prophylaxis, 1 developed C.krusei peri-hepatic abscess and 1 had suspected Candida subhepatic abscess due to elevated serum β-d-Glucan. The 6 patients without ARC screen did not develop post-LT Candida infection.The median time to post-LT Candida infection was 25 days (range 24-26). The median number of risk factors among 3 patients with post-LT Candida infection was 2 vs. 1 among 31 patients without post-LT Candida infection.
*Conclusions: Screening for ARC may assist in selecting appropriate antifungal prophylaxis for LT patients but it may not be sufficient to prevent infection in those with multiple risk factors for post-transplant Candida infection. Larger studies are required to evaluate further the role of ARC screening in antifungal stewardship in LT.
To cite this abstract in AMA style:
Patel KK, Azar MM, Koff A, Belfield K, Peaper DR, Topal J, Malinis M. The Role of Pre-transplant Rectal Screening for Azole-resistant Candida Species in Liver Transplant Candidates [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/the-role-of-pre-transplant-rectal-screening-for-azole-resistant-candida-species-in-liver-transplant-candidates/. Accessed February 8, 2026.« Back to 2021 American Transplant Congress