Clinical Outcomes Associated with Induction Therapy Regimens for Kidney Transplantation in Children: A NAPRTCS and PHIS Collaborative Report
1Pediatric Nephrology, Cedars-Sinai Medical Center, Los Angeles, CA, 2Nephrology Institute, Schneider Children's Medical Center, Tel Aviv, Israel, 3Nephrology, Boston Children's Hospital, Boston, MA, 4Children's Hospital Association, Lenexa, KS
Meeting: 2021 American Transplant Congress
Abstract number: 279
Keywords: Glomerular filtration rate (GFR), Graft survival, Pediatric
Topic: Clinical Science » Kidney » Kidney Immunosuppression: Induction Therapy
Session Information
Session Name: Kidney Immunosuppression
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 7, 2021
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:30pm-6:35pm
Presentation Time: 6:30pm-6:35pm
Location: Virtual
*Purpose: Choice of induction agent at the time of pediatric kidney transplant (KTx) is often based upon patient characteristics and center practice. There are limited large scale comparisons of induction agents used for pediatric KTx. We evaluated clinical outcomes and costs based on induction therapy among children in the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry and the Pediatric Health Information System (PHIS) database.
*Methods: Retrospective study of merged data from the NAPRTCS registry and PHIS database between 1999-2019. Participants were grouped by induction agent: no induction, IL2 RB only, rATG/ALG, alemtuzumab. Unadjusted outcomes included graft failure, estimated GFR (eGFR) at 1, 3, and 5-year post-KTx and index hospitalization cost (IHC). Subgroup analysis evaluated graft failure and eGFR at 1, 3, and 5-year post-KTx for deceased donor kidney transplant (DDKT) vs. living donor kidney transplant (LDKT) between 2009-2019. Categorical outcomes were compared across induction therapy groups using a chi-square test for association; continuous variables were compared using a Kruskal-Wallis test.
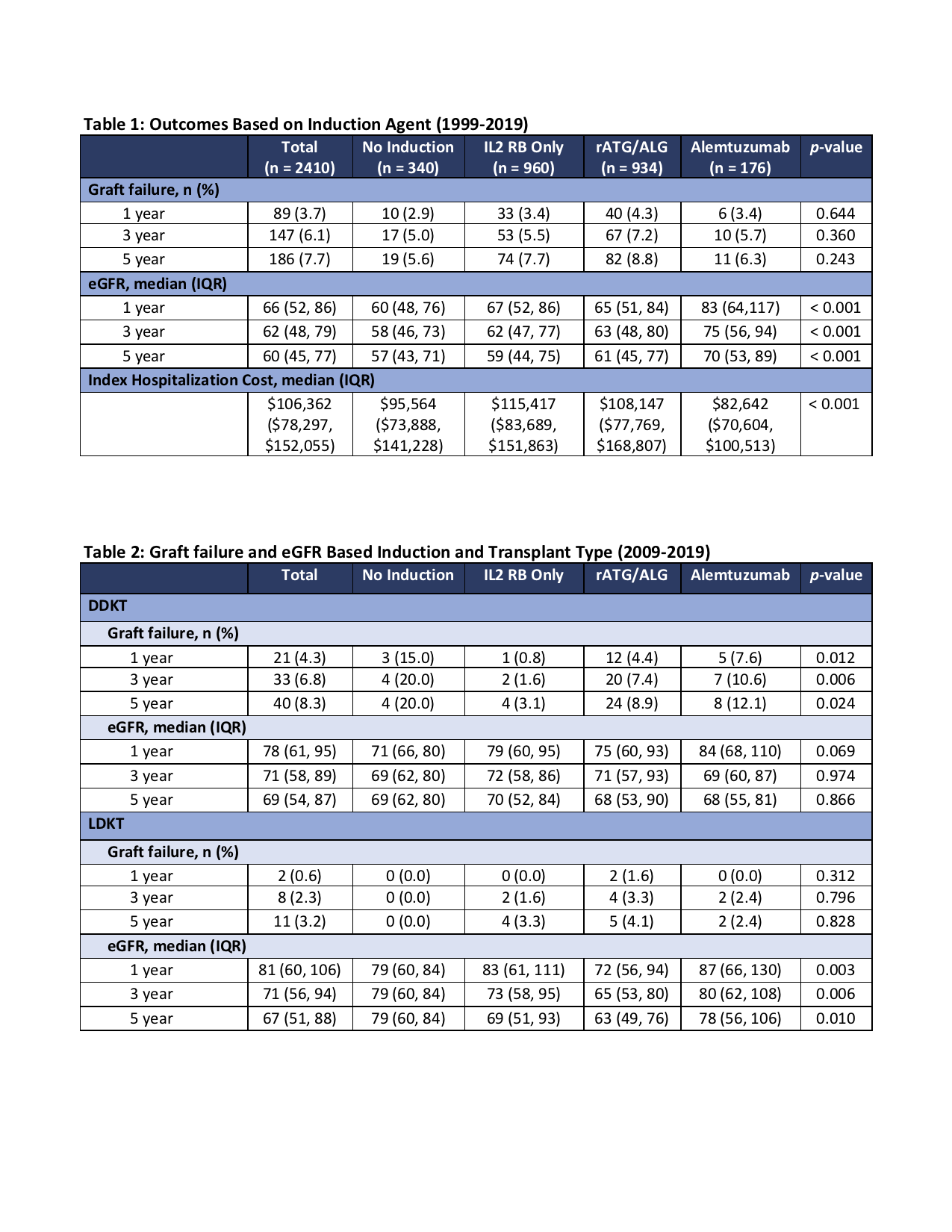
*Results: 2410 KTx with data in both NAPRTCS and PHIS were included in the analysis. 340 subjects (14.1%) received no induction, 960 (39.8%) received IL2 RB only, 943 (39.1%) received rATG/ALG (164 of these also received IL2 RB), and 176 (7.3%) received alemtuzumab. Table 1 highlights the graft failure rate, eGFR, and IHC for those transplanted between 1999-2019. Subset analysis of graft failure and eGFR in those who received DDKT vs. LDKT between 2009-2019 are shown in Table 2 (3.8% no induction, 30.9% IL2 RB, 49.8% rATG/ALG, 15.5% alemtuzumab).
*Conclusions: Over the last two decades IL2 RB and rATG/ALG are the more frequently used induction agents at the time of pediatric KTx, however, there is no difference in graft survival at 1, 3, and 5-years post-KTx based on the induction agent used. Alemtuzumab appears to be associated with higher eGFR at the same time points and appears to be associated with lower IHC. Among DDKT performed between 2009-2019, it appears those without induction had higher rates of graft failure, while eGFR was similar among the induction groups at all three time points. Among LDKT, graft failure was similar but recipients with alemtuzumab induction had higher eGFR across all time points.
To cite this abstract in AMA style:
Pizzo H, Erez DLevy, Rodig NM, Richardson T, Somers M. Clinical Outcomes Associated with Induction Therapy Regimens for Kidney Transplantation in Children: A NAPRTCS and PHIS Collaborative Report [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-outcomes-associated-with-induction-therapy-regimens-for-kidney-transplantation-in-children-a-naprtcs-and-phis-collaborative-report/. Accessed January 13, 2026.« Back to 2021 American Transplant Congress