Safety of Sars-cov-2 Mrna Vaccines in Solid Organ Transplant Recipients
Johns Hopkins School of Medicine, Baltimore, MD
Meeting: 2021 American Transplant Congress
Abstract number: LB 1
Keywords: COVID-19, Safety, Vaccination
Topic: Clinical Science » Infectious Disease » COVID-19
Session Information
Session Name: Late-Breaking: COVID-19
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 7, 2021
Session Time: 4:30pm-5:30pm
 Presentation Time: 4:30pm-4:35pm
Presentation Time: 4:30pm-4:35pm
Location: Virtual
*Purpose: The safety of SARS-CoV-2 mRNA vaccines in solid organ transplant recipients (SOTRs) remains unknown. We investigated adverse events in SOTRs who received these mRNA vaccines.
*Methods: We studied SOTRs between 12/16/2020 – 2/10/2021 who received at least one dose of a vaccine. Vaccine reactogenicity within one week following the first or second dose was self-reported via an interactive, online platform.
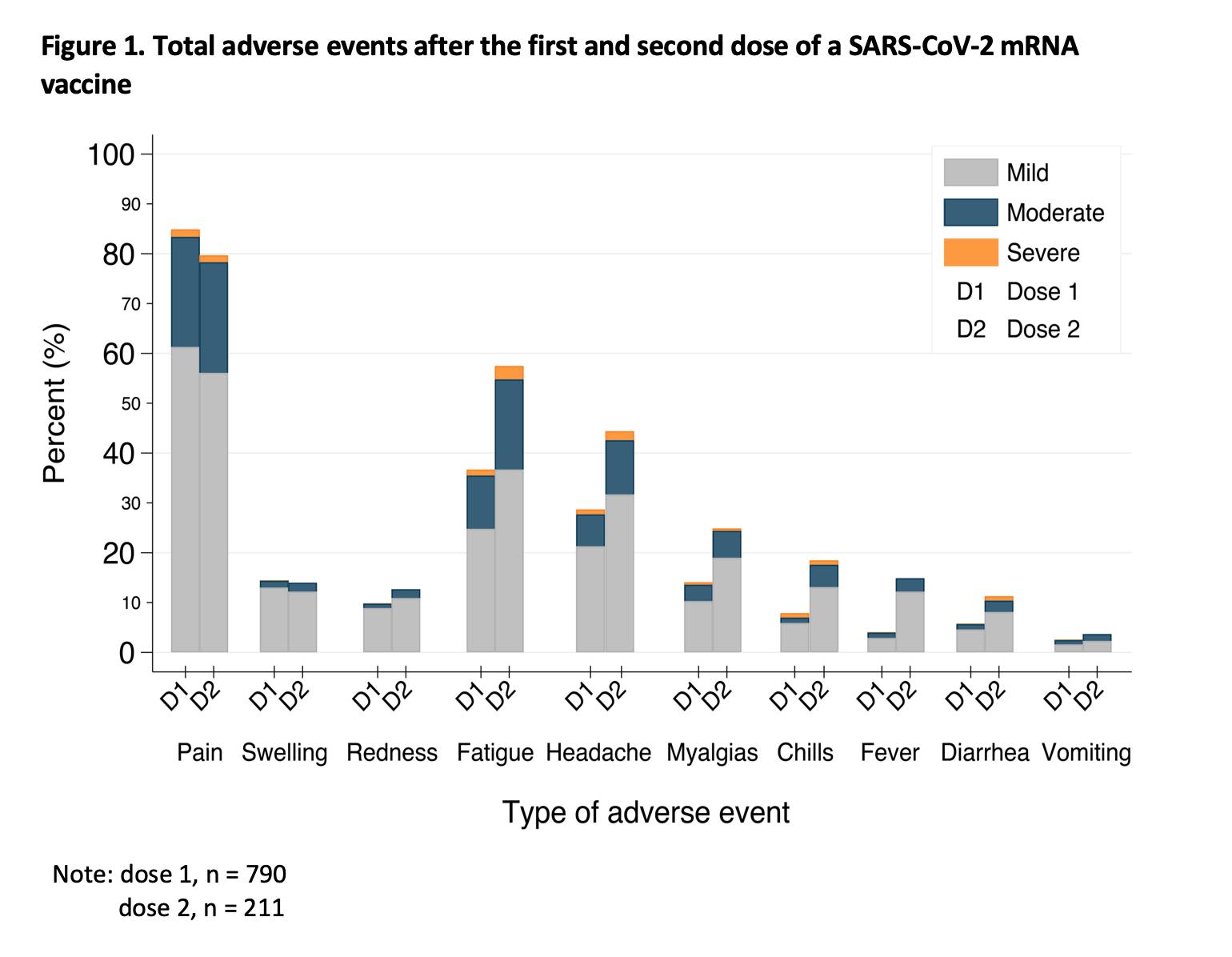
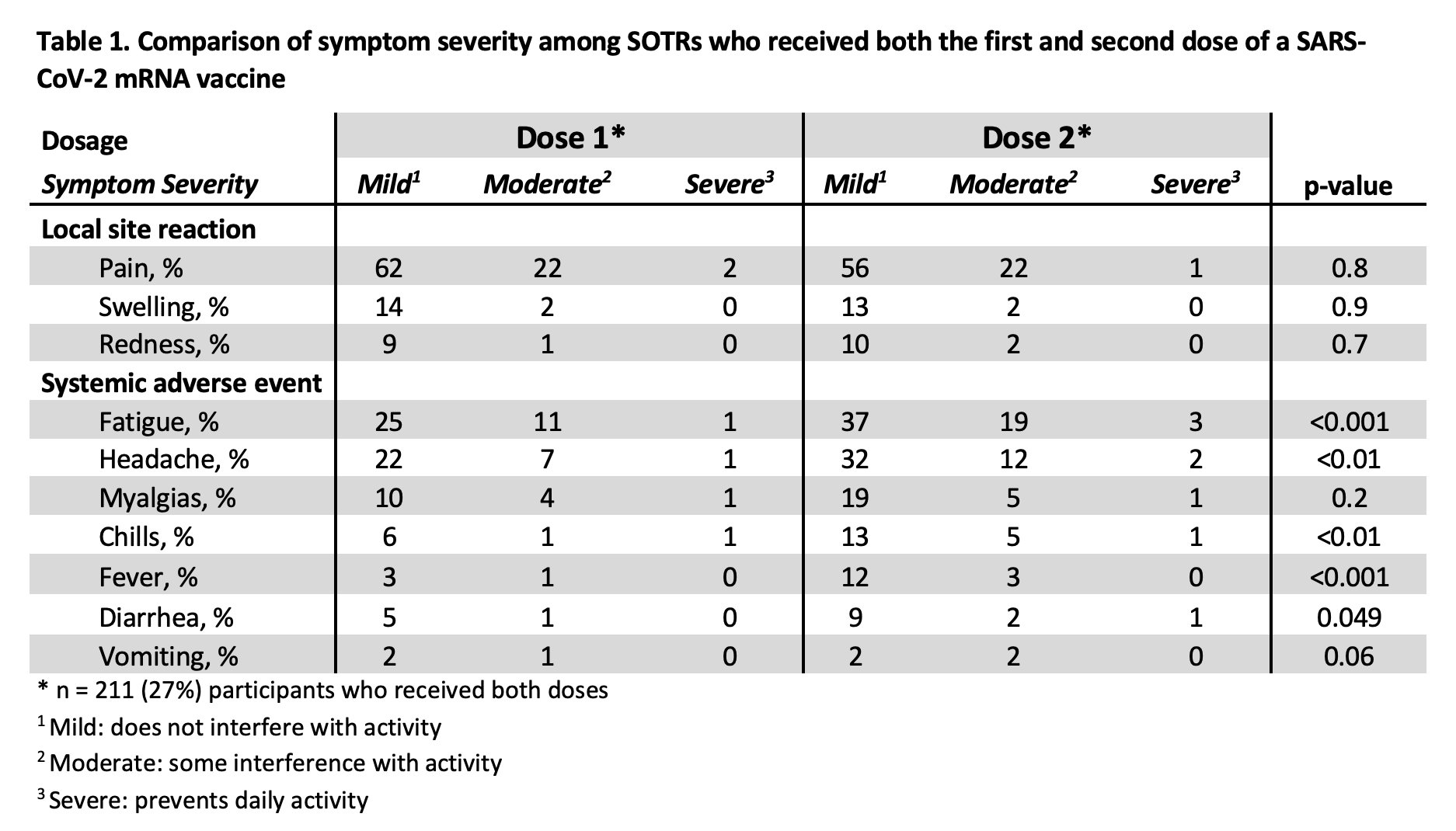
*Results: A total of 790 SOTRs received either the Pfizer/BioNTech (49%) or Moderna (51%) vaccine. Most participants have, thus far, received only one dose, but 211 (27%) received both doses. The median (IQR) age was 58 (43-68), with 57% female, 90% White, and 81% college educated. Organs transplanted include kidney (56%), liver (20%), and heart (16%), with a median (IQR) of 6 (3-13) years since transplantation. There were no reports of new COVID-19 infection, acute rejection, anaphylaxis requiring epinephrine, or new neurological conditions such as Guillain-Barré or Bell’s palsy. Overall, moderate to severe local and systemic adverse reactions remained low (Figure 1). Comparison between the first and second dose showed that moderate to severe systemic adverse reactions, while uncommon, were higher after the second dose, including fatigue (22% vs 12%, p<0.001), headache (14% vs. 8%, p<0.01), chills (6% vs. 2%, p<0.01), and fever (3% vs. 1%, p<0.001)(Table 1).
*Conclusions: In our observational cohort, there were no reports of new COVID-19 infection, acute rejection, anaphylaxis requiring epinephrine, or new neurological conditions following SARS-CoV-2 mRNA vaccination. While uncommon, moderate to severe systemic adverse reactions were higher after the second dose. Thus far, there are no large safety concerns for SARS-CoV-2 mRNA vaccines in SOTRs.
To cite this abstract in AMA style:
Ou M, Boyarsky B, Motter J, Greenberg R, Teles A, Ruddy J, Krach M, Werbel W, Avery R, Massie A, Segev D, Garonzik-Wang J. Safety of Sars-cov-2 Mrna Vaccines in Solid Organ Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-of-sars-cov-2-mrna-vaccines-in-solid-organ-transplant-recipients/. Accessed February 3, 2026.« Back to 2021 American Transplant Congress