Development and Durability of Sars-cov-2 Antibodies Among Solid Organ Transplant Recipients
B. Boyarsky, M. Ou, R. Greenberg, M. Krach, R. Wang, T. P. Chiang, W. Werbel, R. Avery, W. Clarke, A. Tobian, A. Massie, D. Segev, J. Garonzik-Wang
Johns Hopkins, Baltimore, MD
Meeting: 2021 American Transplant Congress
Abstract number: 22
Keywords: Antibodies, Vaccination
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: COVID-19 Session 1
Session Type: Rapid Fire Oral Abstract
Date: Saturday, June 5, 2021
Session Time: 4:30pm-5:30pm
 Presentation Time: 4:45pm-4:50pm
Presentation Time: 4:45pm-4:50pm
Location: Virtual
*Purpose: The response to SARS-CoV-2 may be blunted in transplant recipients, impacting reinfection risk, treatment selection, and vaccine protocols. We quantified antibody response and durability after COVID-19 in solid organ transplant recipients (SOTRs).
*Methods: SOTRs with PCR-confirmed COVID-19 were recruited through the EMR August 21-October 15, 2020. Participants underwent at-home blood sampling with the TAPTM Blood Collection Device, Second Edition (7SBio, Medford, MA). Serum samples were screened using Elecsys® anti-SARS-CoV-2 immunoassay (Roche), which uses a recombinant protein representing the nucleocapsid antigen. Confirmatory testing was performed using EUROIMMUN anti-SARS-CoV-2 enzyme-linked immuosorbent assay (ELISA) for semi-quantitative detection of IgG antibodies to spike protein (anti-S1-IgG), a likely correlate of neutralizing immunity.
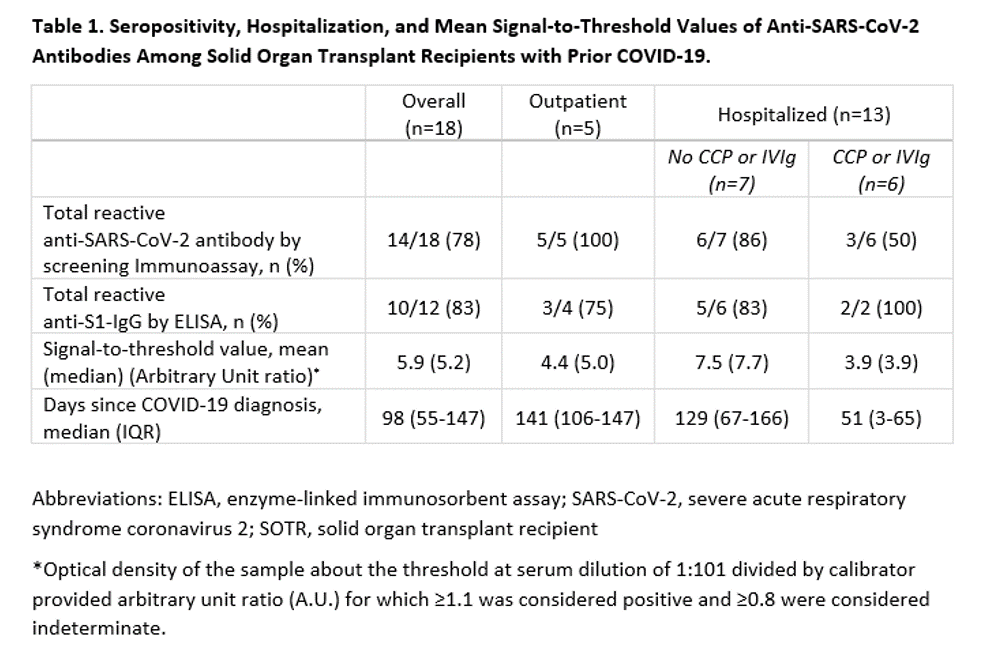
*Results: Eighteen SOTRs were studied, for whom COVID-19 occurred at a median of 6 years (IQR 2-9) post-transplant. Median age was 56 years (IQR 42-63); 56% were female; 33% were Black and 11% were Hispanic. Most participants (89%) had experienced COVID-19 symptoms; 72% were hospitalized. Among those hospitalized, 15% were admitted to the ICU and 8% were mechanically ventilated. COVID-19 convalescent plasma (CCP) was administered to 3 kidney and 2 lung recipients. At median 98 days (IQR 55-147) after COVID-19 diagnosis, 78% had reactive screening immunoassays (Table 1). Of the four patients with non-reactive immunoassays, 2 were the lung recipients treated with CCP and 1 was the kidney recipient receiving IVIg. Of those who screened positive, anti-S1-IgG was detectable in 83%. SOTRs who received CCP and/or IVIg were less likely to develop anti-S1-IgG and had lower antibody levels.
*Conclusions: We found antibody levels suggestive of neutralizing immunity in the majority of participants. However, those who were administered CCP and/or IVIg were less likely to mount a durable immune response. This raises the possibility that exogenous antibody preparations may blunt durable antibody formation. We observed a significant association between more severe disease and higher antibody levels. Seropositivity might decline over time; however, we were unable to distinguish between impaired production or rapid decrement. Our findings are important for individuals with compromised immune systems, whether deliberately for conditions like organ transplantation and cancer, or naturally in the elderly, frail, and autoimmune populations.
To cite this abstract in AMA style:
Boyarsky B, Ou M, Greenberg R, Krach M, Wang R, Chiang TP, Werbel W, Avery R, Clarke W, Tobian A, Massie A, Segev D, Garonzik-Wang J. Development and Durability of Sars-cov-2 Antibodies Among Solid Organ Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/development-and-durability-of-sars-cov-2-antibodies-among-solid-organ-transplant-recipients/. Accessed January 7, 2026.« Back to 2021 American Transplant Congress