Avoiding Surveillance Biopsy: How? When? Now!
1School of Medicine, University of Alabama, Birmingham, Birmingham, AL, 2Transplant Genomics Inc., Mansfield, MA
Meeting: 2020 American Transplant Congress
Abstract number: C-336
Keywords: Biopsy, Kidney transplantation, Rejection, Renal dysfunction
Session Information
Session Name: Poster Session C: Biomarkers, Immune Assessment and Clinical Outcomes
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: The TruGraf™ blood test measures a specific gene expression signature in the peripheral blood mononuclear cells to enable noninvasive serial assessment of kidney transplant recipients (KTRs) with stable renal function, ruling out subclinical acute rejection (subAR) with a high degree of confidence. The objective of this study was to correlate the TruGraf™ test with the histology findings on a 6-month surveillance biopsy (SBx), and evaluate follow-up of patient outcomes for 1 year after the SBx.
*Methods: 116 KTRs were consecutively enrolled in a single-center observational study where SBx is standard of care at 6 months post-transplant. Serum creatinine (SCr) and TruGraf™ testing were collected following informed consent prior to biopsy. TruGraf™ results (Tx or not-Tx) were compared with histology using Banff 2017 criteria to assess concordance. Subsequent clinical follow-up was recorded. Clinical utility of TruGraf™ results was determined based on a questionnaire with the investigators.
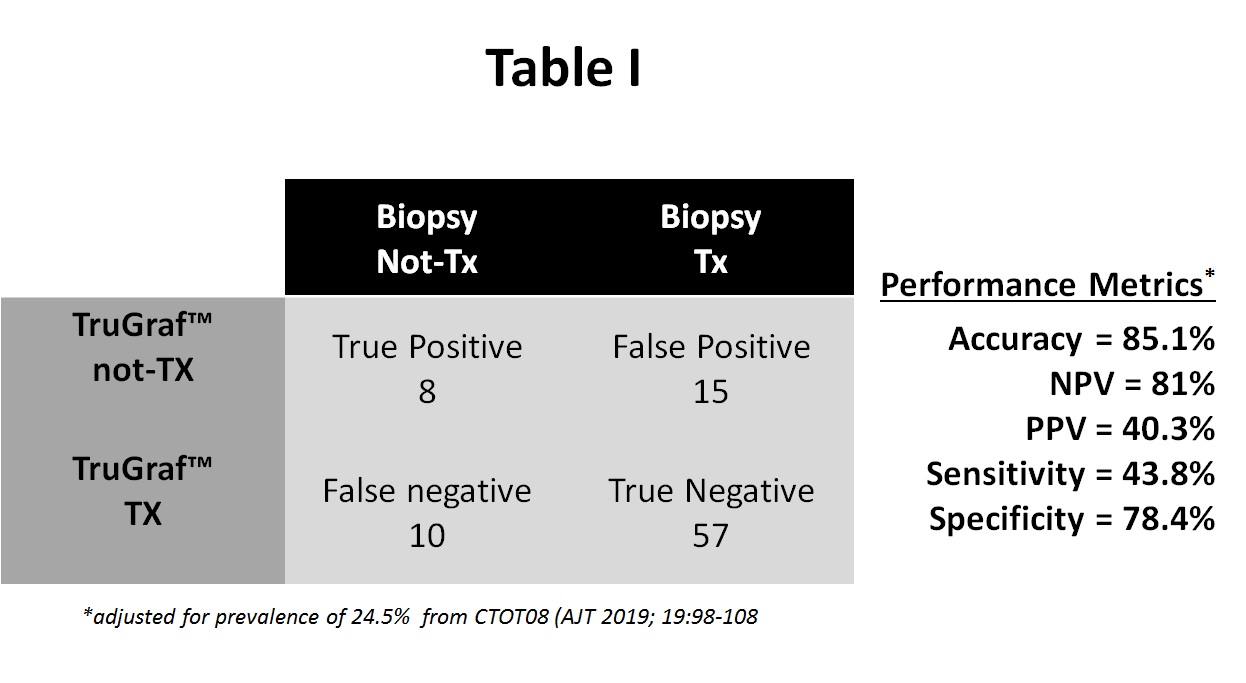
*Results: Of the 116 enrollees, 26 were excluded for either absent allograft biopsy (n=17) or quality control issues with the test (n=9), leaving 90 KTRs in this cohort. 66% received deceased donor kidneys, 58% were African American, and 59% male. 60 have been followed for at least 1 year post-transplant, with a mean SCr 1.38±0.41 mg/dL. In the follow-up to date, there were no subsequent acute rejections but there were 2 deaths with functioning allografts. The overall concordance of TruGraf™ results compared with histology is shown in table I. TruGraf™ correctly identified 57/90 (63.3%) of patients as true negative (Tx), all of whom could have been avoided SBx. In addition, the test identified 8/90 (8.8%) of subjects with a true positive (not-Tx) result where a biopsy was justified. Conversely, an unnecessary biopsy would still be performed on 15/90 subjects (16.7%) with a false positive TruGraf™, and silent subAR would have been missed in 9% of subjects despite stable renal function. Clinical utility was 77% with results supporting patient management decisions.
*Conclusions: We demonstrate the implementation of TruGraf™ testing in a “real-world” cohort at the time of SBx. We identified a significant proportion of KTRs that could have avoided SBx. Further studies are focusing on the potential applications of TruGraf™ including reducing reliance on SBx, proactive serial testing, and patient stratification into those with stable transplant outcomes in the first year after transplantation.
To cite this abstract in AMA style:
Ang A, Schieve C, Rose S, Kew C, First MR, Mannon RB. Avoiding Surveillance Biopsy: How? When? Now! [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/avoiding-surveillance-biopsy-how-when-now/. Accessed February 5, 2026.« Back to 2020 American Transplant Congress