Glecaprevir-Pibrentasvir Effect on Tacrolimus Troughs in Abdominal Transplant Recipients
M. Chunduru, K. Schnelle, H. Winters, O. Witkowsky, L. Von Stein
The Ohio State Wexner Medical Center, Columbus, OH
Meeting: 2020 American Transplant Congress
Abstract number: C-201
Keywords: Drug interaction, Hepatitis C, Viral therapy
Session Information
Session Name: Poster Session C: Non-Organ Specific: Viral Hepatitis
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Glecaprevir-pibrentasvir (GP) is one of several pangenotypic therapies available for the treatment of hepatitis C. Historical use of ritonavir based hepatitis C therapies increased tacrolimus concentrations due to known strong inhibition of CYP3A4. GP is noted to be a weak inhibitor of CYP3A4, but insufficient data exist regarding its impact on tacrolimus troughs. This study describes the effect of GP on tacrolimus troughs in abdominal transplant recipients.
*Methods: A retrospective analysis of liver and/or kidney patients transplanted between November 2017 and July 2019 receiving GP post-transplant was conducted. Patients were excluded if they had not completed 12 weeks of therapy with GP. Tacrolimus troughs were collected prior to and during GP administration per institution lab protocol.
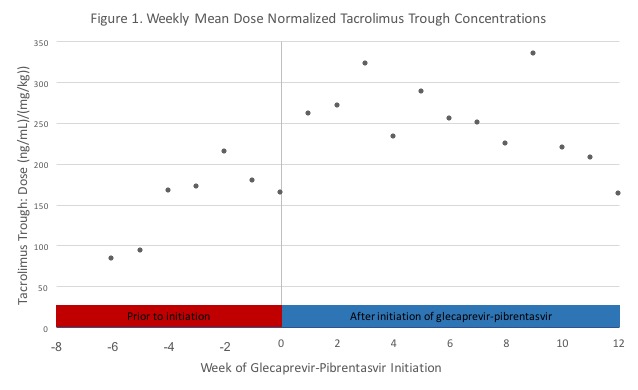
*Results: Fourteen recipients were included in this analysis: kidney (n=5, 35.7%), liver (n=8, 57.1%), simultaneous liver and kidney (n=1, 7.1%). Time to GP initiation was a mean of 153 days (SD +/- 100.4) post-transplant. The majority of patients had hepatitis C prior to transplant while others received a hepatitis C nucleic acid test positive organ (64.3% versus 35.7%). Figure 1 shows the weekly mean dose normalized tacrolimus trough concentrations of recipients prior to GP initiation and during GP therapy. The mean percent change in dose normalized tacrolimus troughs (ng/ml/dose) after GP initiation was an increase of 24% in kidney alone transplant recipients and 61% in liver transplant recipients. With regard to tacrolimus dose adjustments, kidney alone recipients had a mean decrease of 15%, while liver recipients had a mean decrease of 28%. Of note, 2 of 5 kidney alone recipients and 3 of 9 liver recipients had no dose changes. Eleven patients (4 kidney alone recipients and 7 liver recipients) had supratherapeutic tacrolimus (>10ng/ml) troughs while on GP. Of these patients, 2 kidney alone recipients also had supratherapeutic levels immediately prior to initiation of GP.
*Conclusions: Based on these data, using glecaprevir-pibrentasvir without preemptive dose adjustment of tacrolimus is reasonable in kidney and liver transplant recipients, but close tacrolimus therapeutic drug monitoring should be performed while on GP. A larger study may help guide the timing and extent of dose adjustment of tacrolimus in abdominal transplant recipients.
To cite this abstract in AMA style:
Chunduru M, Schnelle K, Winters H, Witkowsky O, Stein LVon. Glecaprevir-Pibrentasvir Effect on Tacrolimus Troughs in Abdominal Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/glecaprevir-pibrentasvir-effect-on-tacrolimus-troughs-in-abdominal-transplant-recipients/. Accessed February 17, 2026.« Back to 2020 American Transplant Congress