What is the Significance of Denovo Donor Specific Antibodies in Recipients of Simultaneous Liver-Kidney Transplants?
University of Wisconsin, Madison, WI
Meeting: 2020 American Transplant Congress
Abstract number: C-123
Keywords: Antibodies, Liver transplantation
Session Information
Session Name: Poster Session C: Liver - Kidney Issues in Liver Transplantation
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: There is limited information about the utility of donor-specific HLA antibody (DSA) testing and the role of protocol kidney biopsy for de-novo donor-specific antibodies (dnDSA) among simultaneous liver and kidney (SLK) transplant recipients.
*Methods: We analyzed our SLK recipients transplanted between 01/2005 and 12/2017 who had DSA tested post-transplant. Patients were divided into two groups based on whether they developed dnDSA post-transplant (dnDSA+) or not (dnDSA-). Patients were excluded if DSA was not tested post SLK. Kidney graft rejection ± 60 days of dnDSA and kidney graft survival were the primary endpoints.
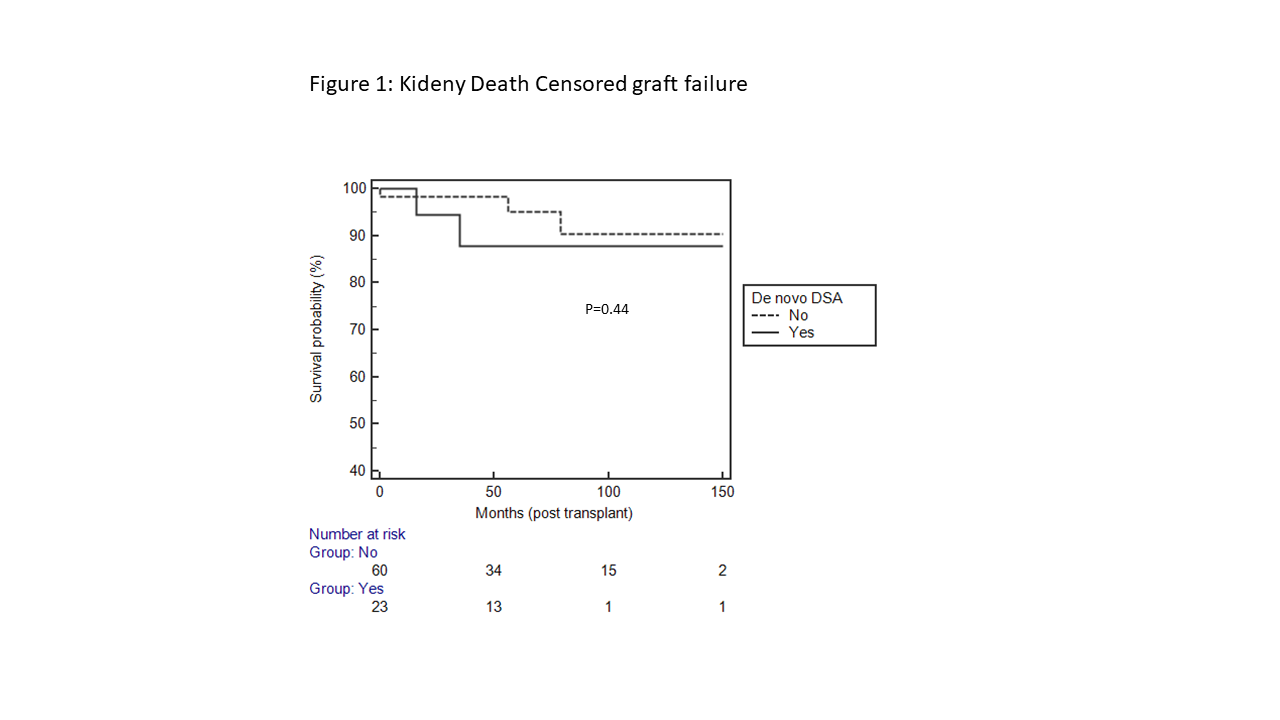
*Results: A total of 91 SLK were transplanted during that period, of which 83 fulfilled our selection criteria. Of those, 23 were in dnDSA+ group and 60 in dnDSA-. 36 recipients had pre-transplant DSA, of which 6 developed dnDSA. None of the baseline characteristics were different between the groups. The mean interval from transplant to the detection of dnDSA was 34 ± 41 months. 22 of 23 dnDSA+ patients had DSA against class II HLA and only one had dnDSA against class I. 15 recipients underwent kidney biopsy after detection of dnDSA, of which 6 were clinically indicated due to rise in serum Cr or proteinuria- none of these 6 had rejection. Of the 9 who had clinically stable kidney function but had a protocol biopsy only due to dnDSA, 6 had rejection (2 antibody mediated, 1 cellular, 3 mixed). Of these 9 patients with dnDSA, in 4 DSA was monitored in routine clinic visit, in one per protocol at 3 months post transplant, in 3 due to liver graft dysfunction and in one due to being on suboptimal immunosuppresive medication due to severe infection. 6 patients underwent liver biopsy, of which 4 had cellular rejection. Of these 4 rejections, one was in the protocol kidney biopsy group, 2 in the clinically indicated kidney biopsy group and one patient underwent isolated liver biopsy without kidney biopsy. At last follow up, dnDSA was not associated with death censored graft failure of kidney (Figure 1) or liver.
*Conclusions: In SLK recipients with dnDSA and stable kidney function, we found that 6 of 9 who were biopsied had subclinical kidney rejection. Possibly in part because of the identification of subclinical rejection and early treatment, outcomes were favorable despite the rejections. Although our study was limited by small sample size, it suggests that DSA monitoring and protocol biopsy for dnDSA may be useful in SLK recipients, as has been previously demonstrated for recipients of kidney transplants alone.
To cite this abstract in AMA style:
Parajuli S, Aziz F, Blazel J, Muth B, Garg N, Mohamed M, Rice J, Mezrich J, Hidalgo L, Mandelbrot D, Im G, Lee B. What is the Significance of Denovo Donor Specific Antibodies in Recipients of Simultaneous Liver-Kidney Transplants? [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/what-is-the-significance-of-denovo-donor-specific-antibodies-in-recipients-of-simultaneous-liver-kidney-transplants/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress