Basiliximab Induction in the Obese: One Size Does Fit All
1Pharmacy Practice, University of Illinois Hospital and Health Sciences System, Chicago, IL, 2Pharmacy, Northwestern Memorial Hospital, Chicago, IL, 3Surgery, University of Illinois Hospital and Health Sciences System, Chicago, IL
Meeting: 2020 American Transplant Congress
Abstract number: C-005
Keywords: Induction therapy, Kidney transplantation, Obesity, Pharmacokinetics
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
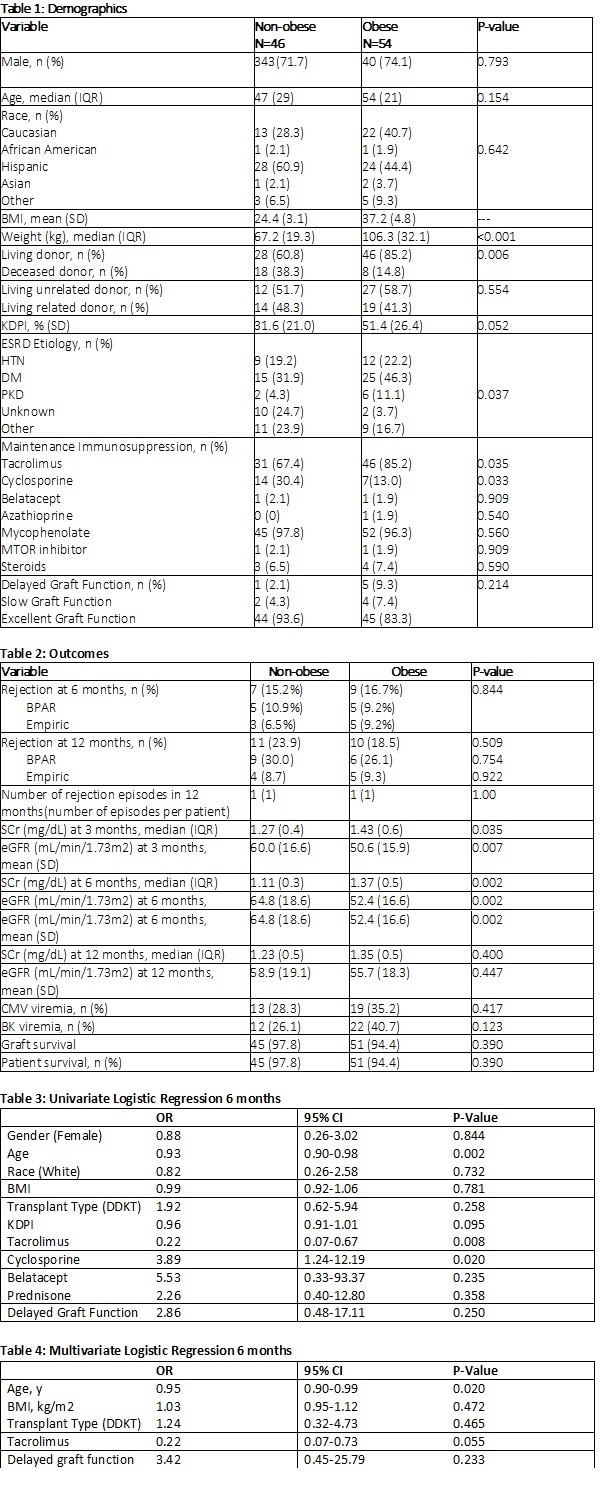
*Purpose: Surgical advancements allow more obese (BMI> 30 kg/m2) patients to receive a renal transplant. The original clinical and pharmacokinetic (PK) trials in transplantation immunosuppression did not include overweight subjects. The initial basiliximab PK studies showed a small, but significant, correlation between weight and PK properties of basiliximab. This study aims to compare incidence of rejection in non-obese versus obese patients following renal transplant with basiliximab induction.
*Methods: Renal transplant recipients between 1/1/15 and 9/1/18 were retrospectively identified for analysis. The primary outcome assessed the incidence of rejection at 6 months post-transplant in non-obese compared to obese subjects following basiliximab induction. Secondary outcomes include: number of rejection episodes, eGFR and serum creatinine (SCr) at 3, 6, and 12 months, and graft survival at 12 months. Additional secondary safety outcomes include CMV viremia, BK viremia, and patient survival at 12 months.
*Results: A total of 100 patients were analyzed. Demographics were similar between groups (Table 1). There was no difference in the incidence of rejection at 6 months post-transplant in non-obese or obese patients (15.2% vs 16.7%, p=0.844, Table 2). The number of rejections, rejection rate at 12 months, and severity of rejection were similar between the two groups. There was a numerically higher rate of obese patients who experienced delayed graft function (DGF) (2.1% vs 9.3%, p=0.21). Non-obese patients had significantly improved SCr and eGFR at 3 months and 6 months (Table 2). There was no difference in graft or patient survival (Table 2). Multivariate logistic regression showed BMI was not an independent risk factor for rejection (p=0.47, Table 3).
*Conclusions: There was no difference in rate of rejection at 6 or 12 months post-transplant between the obese and non-obese cohort. eGFR was significantly higher in the non-obese cohort at both 3- and 6-months post-transplant. Despite these early differences, the 12-month eGFR was similar between groups. These findings reassure the use of basiliximab in the obese and prevent a need to switch to a depleting agent, which can incur higher rates of infection. Given the study results, basiliximab is an effective method of induction for the low risk, obese kidney transplant patient population.
To cite this abstract in AMA style:
Reticker AD, Choi D, Lichvar AB, Kane C, Tzvetanov I, Benedetti E, Benken JJ. Basiliximab Induction in the Obese: One Size Does Fit All [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/basiliximab-induction-in-the-obese-one-size-does-fit-all/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress