Relationship between Tacrolimus Intrapatient Variability and Medication Adherence in Kidney Transplant Recipients
A. D. Leino, K. Abuls, J. L. Rodgers, K. Kuntz
The Ohio State University Wexner Medical Center, Columbus, OH
Meeting: 2020 American Transplant Congress
Abstract number: B-240
Keywords: Calcineurin, Kidney, Monitoring, Pharmacokinetics
Session Information
Session Name: Poster Session B: Psychosocial and Treatment Adherence
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Intrapatient variability (IPV) has been established as a risk factor for poor post-transplant outcomes. IPV is frequently proposed to be related to nonadherence but is not well studied. The purpose of this study was to evaluate the relationship between IPV and adherence in kidney transplant recipients.
*Methods: This was a retrospective cohort analysis of kidney recipients transplanted between August 1, 2016 and June 18, 2018. Patients were included if initiated and maintained on tacrolimus-based immunosuppression and had an adherence assessment with a pharmacist at approximately 12 months post-transplant. Nonadherence was defined as a composite endpoint including verbal patient self-report of missed or significantly late doses, documented pharmacist concern for nonadherence, and/or completion of <80% of protocol lab monitoring at 12 months. IPV was calculated as the coefficient of variation (CV=SD/mean*100) using all tacrolimus levels from 3-12 months post-transplant. The relationship between adherence status and CV was evaluated using multiple linear regression. CV was log-transformed for analysis to meet the assumptions of the model.
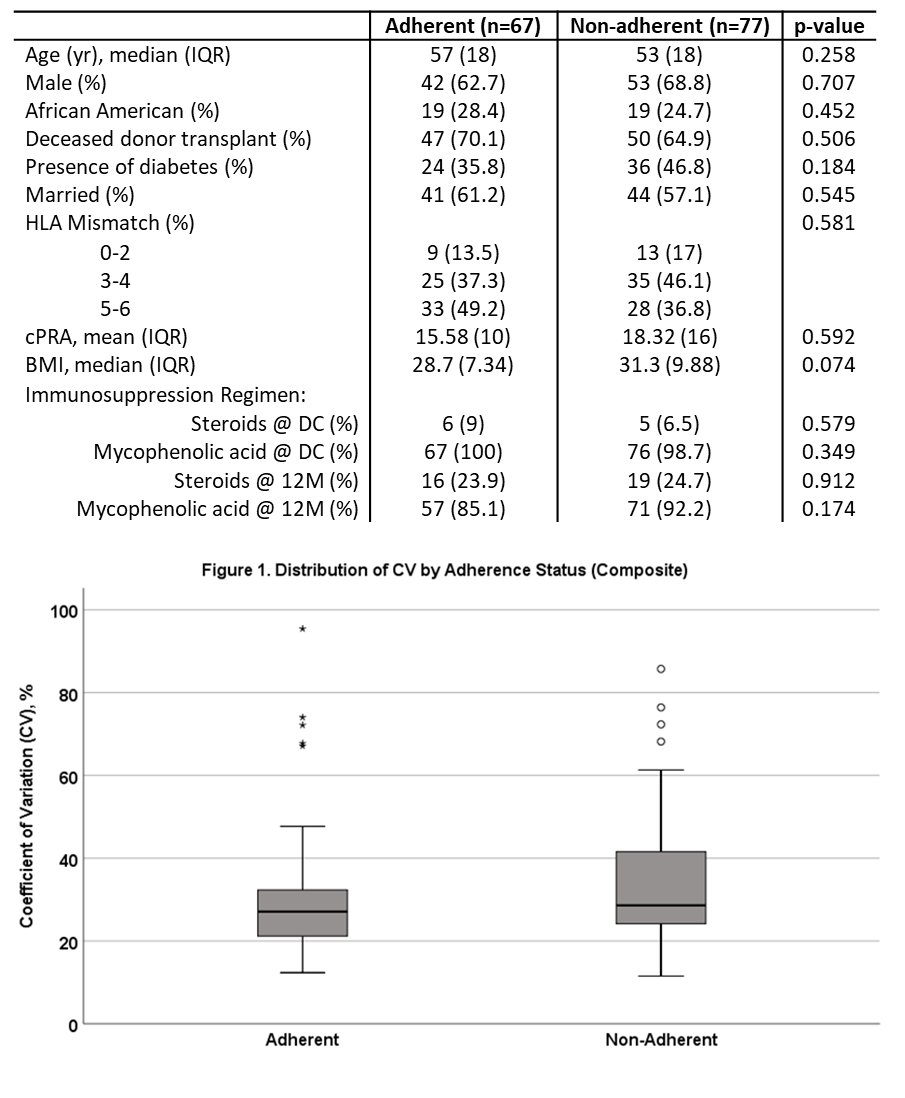
*Results: 144 patients were included in the analysis. A high level of nonadherence was noted with 53.5% demonstrating nonadherence on 1 or more measures at 12 months. No baseline characteristics were significantly different between adherent and non-adherent patients (Table 1). Median CV was 27.1% for adherent patients and 28.3% for nonadherent patients (p=0.125, Figure 1). The only significant predictor of CV was the number of tacrolimus dose changes; each dose change resulted in an estimated mean CV increase of 6% (p= 0.004). Subgroup analysis of the 19 patients labeled nonadherent due to the most objective marker, lab monitoring, demonstrated a significant difference in CV (27.1% adherent vs. 35.9% non-adherent, p=0.014). This nonadherent subgroup completed on average only 55% of the required blood draws. There were no differences in de novo DSA development or biopsy proven acute rejection between groups, though all 4 rejection episodes were in the nonadherent group (p=0.058).
*Conclusions: This study did not confirm an association between tacrolimus CV and a composite measure of nonadherence. Subgroup analysis suggests CV may be better suited to identify high levels of nonadherence consistent with failure to complete a large percentage of the recommended medical care.
To cite this abstract in AMA style:
Leino AD, Abuls K, Rodgers JL, Kuntz K. Relationship between Tacrolimus Intrapatient Variability and Medication Adherence in Kidney Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/relationship-between-tacrolimus-intrapatient-variability-and-medication-adherence-in-kidney-transplant-recipients/. Accessed February 6, 2026.« Back to 2020 American Transplant Congress