Viral Kinetics of De Novo HCV Infection and Response to Antiviral Therapy in Liver and Kidney Transplant Recipients Receiving HCV-Viremic Donors
N. Terrault1, J. Burton2, E. Verna3, C. Mobley4, D. Victor4, J. Trotter5, C. Niemann6, R. Rubin7
1University of Southern California, Los Angeles, CA, 2UC, Denver, CO, 3Columbia Universeity, New York, NY, 4Houston Methodist, Houston, TX, 5Baylor University, Dallas, TX, 6UCSF, San Francisco, CA, 7Piedmont Atlanta Hosptial, Atlanta, GA
Meeting: 2020 American Transplant Congress
Abstract number: B-200
Keywords: Donors, marginal, Hepatitis C, Safety, Viral therapy
Session Information
Session Name: Poster Session B: Non-Organ Specific: Viral Hepatitis
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: There is general agreement that expanded use of organs from HCV-viremic donors is desirable, including in recipients without HCV infection. However, the optimal treatment approach has not been defined. We sought to evaluate the kinetics of HCV infection post-transplant comparing kidney and liver transplant recipients of viremic donors and consequences of delayed initiation of antiviral therapy.
*Methods: 6 U.S. transplant programs prospectively enrolled adults listed for primary LT or KT with the following exclusions: re-transplant, HBsAg-positive or HIV infection, multiorgan (except SLK). SOF/VEL was started once viremia was confirmed post-transplant and the patient judged to be clinically stable . The primary endpoints were SVR12 and safety.
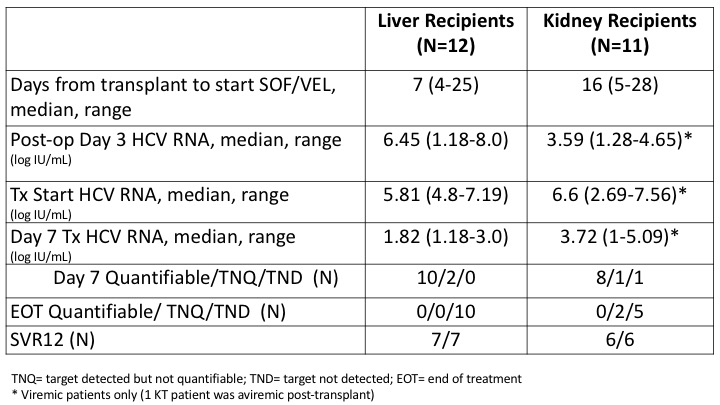
*Results: Of 23 who were transplanted (12 liver, 11 kidney), 22 became viremic post-transplant. Recipients had mean age 54 yrs, 57% male; donors’ mean was 38 years, 78% male, with 74% with confirmed brain death. HCV genotypes were 57% GT1, 26%GT2, 4%GT3 and remainder not determined. Median time from listing for HCV-viremic donor to transplant was 98 and 48 days for liver and kidney recipients, respectively. Median log HCV RNA log10 IU/mL on day 3 was 6.45 in LT and 3.59 in KT; the kinetics of HCV RNA decline are shown in Table. The median time to start of antiviral therapy was 7 days for liver and 16 for kidney recipients. N=5 had delay in treatment start beyond day 21. Yet, all recipients reaching the SVR12 timepoint were HCV negative (13/13), including 2 with detectable HCV RNA at end of treatment (both KT recipients). Serious adverse events considered possibly related were: 1 antibody-mediated rejection, 1 biliary sclerosis and 1 cardiomyopathy after LT. There were no instances of HCV-associated kidney injury, BK viremia, or cholestatic hepatitis.
*Conclusions: All transmitted infections were detectable by day 3, with different viral kinetics in KT versus LT. Delays in starting SOF/VEL were associated with higher VL at start and day 7 of treatment but all attained SVR12. Safety was excellent, but adverse immunologic events viewed as possibly related occurred.
To cite this abstract in AMA style:
Terrault N, Burton J, Verna E, Mobley C, Victor D, Trotter J, Niemann C, Rubin R. Viral Kinetics of De Novo HCV Infection and Response to Antiviral Therapy in Liver and Kidney Transplant Recipients Receiving HCV-Viremic Donors [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/viral-kinetics-of-de-novo-hcv-infection-and-response-to-antiviral-therapy-in-liver-and-kidney-transplant-recipients-receiving-hcv-viremic-donors/. Accessed February 5, 2026.« Back to 2020 American Transplant Congress