BAFF Inhibition to Prevent Antibody Rebound Following Proteasome Inhibitor-Based Desensitization: A Randomized, Controlled Trial
1U Cincinnati, Cincinnati, OH, 2Surgery, U Cincinnati, Christ Hospital, Cincinnati, OH, 3Christ Hospital, Cincinnati, OH
Meeting: 2020 American Transplant Congress
Abstract number: 568
Keywords: Antibodies, B cells, Bone marrow, Kidney
Session Information
Session Name: Kidney Immunosuppression: Desensitization
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:15pm-3:27pm
Presentation Time: 3:15pm-3:27pm
Location: Virtual
*Purpose: Proteasome inhibitor (PI) therapy reduces bone marrow plasma cells and HLA antibodies (Abs) in renal transplant candidates. However, rebound is common in many patients (pts). Increases in BAFF levels occur after B cell depletion, and may be an important mechanism Ab rebound, which may be reduced by belimumab, a BAFF inhibitor. Results from a RCT of bortezomib desensitization, with or without belimumab, are presented.
*Methods: All pts received 2 cycles of bortezomib (1.3mg/m2) with plasmapheresis before the 1st and 4th dose of each cycle. In group A, no additional treatment was administered. In group B, pts received belimumab 10mg/kg IV on days 0, 10, 28, 37, 65, 94, and 121. Primary endpoints were safety and reduction in immunodominant Ab (iAb), measeured by single antigen beads. cPRA was calculated using a precision PRA calculator and donors required to match (DRTM) were defined as (1/1-cPRA) and fold decrease in DRTM as (DRTM pre-DS/DRTM post-DS).
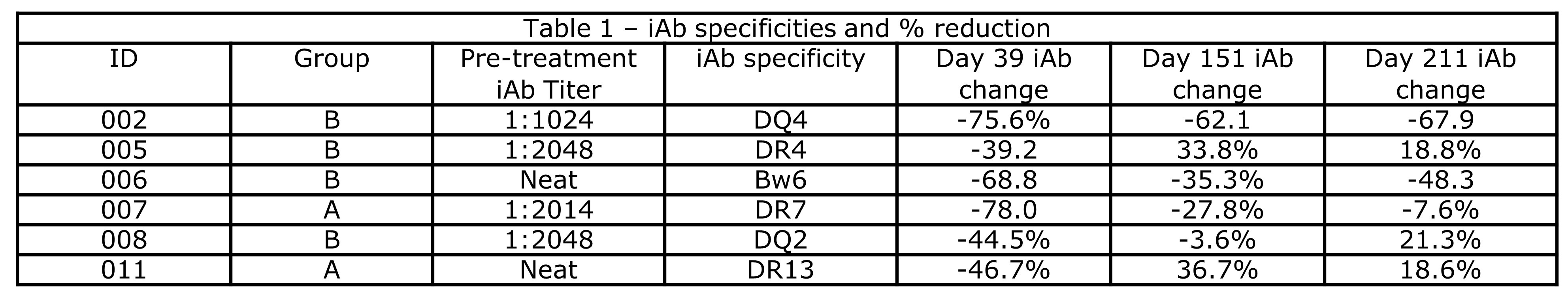
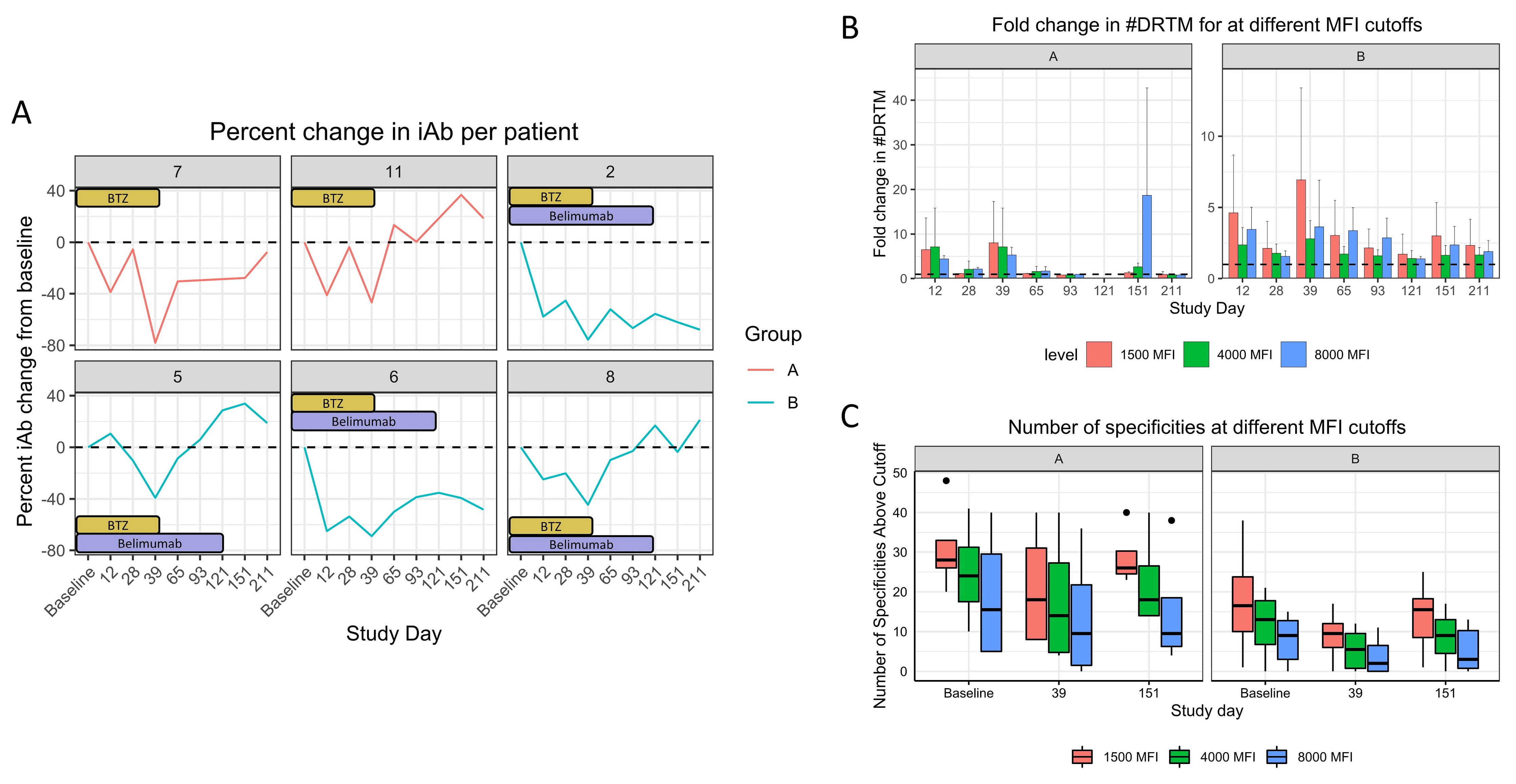
*Results: 10 pts were enrolled and are included in the safety analysis. 2 pts in group A and 4 in group B reached the primary endpoint (others: transplanted [3], adverse event [1], died [1]) and are included in efficacy analysis. Mean age was 46, 70% were Caucasian and female, and median cPRA was 99.41%. Treatment was well tolerated; 2 pts had a grade >3 toxicity. 2 patients reported paresthesia. 5/6 pts had class II iAbs, and 4/6 pts required dilutional analyses due to SAB saturation. All pts had an iAb reduction by day 39 (mean 62.4% and 57.0% for groups A and B respectively), which persisted in 50% in group A and 75% in group B by day 151 (range 3.6% to 62.1%, see Table 1). Figure 1A details individual change in iAb, Figure 1B fold decrease in #DRTM, and 1C change in the number of HLA Abs.
*Conclusions: Belimumab therapy was well tolerated, with all pts having a reduction in iAb and #DRTM. 2/4 pts receiving belimumab had substantial long-term suppression of iAb levels, supporting continued evaluation of belimumab.
To cite this abstract in AMA style:
Tremblay S, Rike-Shields A, Govil A, Cardi M, Brailey P, Alloway R, Woodle E. BAFF Inhibition to Prevent Antibody Rebound Following Proteasome Inhibitor-Based Desensitization: A Randomized, Controlled Trial [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/baff-inhibition-to-prevent-antibody-rebound-following-proteasome-inhibitor-based-desensitization-a-randomized-controlled-trial/. Accessed February 1, 2026.« Back to 2020 American Transplant Congress