Clinical Utility of a Pharmacogenomic Testing Tool among Kidney Transplant Recipients
Kidney/Pancreas Transplant Program, Intermountain Medical Center, Murray, UT
Meeting: 2019 American Transplant Congress
Abstract number: D214
Keywords: Drug interaction, Genomics, Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session D: Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Therapeutic drug monitoring (TDM) for tacrolimus (Tac) is the cornerstone of clinical workflow in the transplant clinic. We report on the clinical implementation of, Rx Match®, a DNA testing platform that measures, analyzes, and interprets a patient’s DNA to determine what medications and dosage selections are appropriate based on genetic information in a kidney transplant recipient population.
*Methods: RxMatch® provides information about CYP2C19, CYP2C9, CYP2D6, CYP3A4, and CYP3A5. In addition to providing information about a gene’s activity this test also provides specific recommendations for medication dosing and expected side effect severity. It also provides information on genes affecting thrombosis risk, including Factor V Leiden and VKORC1 activity. In July 2018 we implemented a new process to screen patients in work-up for kidney transplant with this assay. RxMatch® was obtained by cheek swab in the transplant clinic at the pre-operative visit or during the transplant admission.
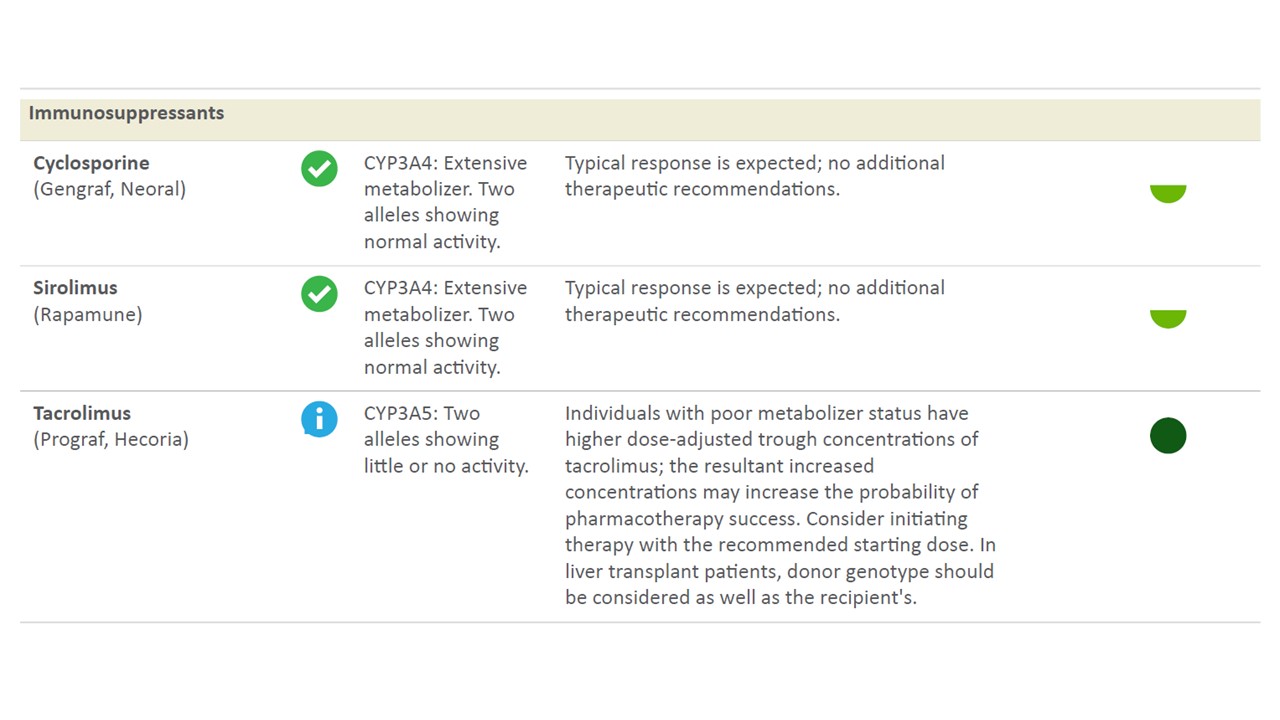
*Results: 14 patients received testing and were included. 7 patients were classified based on CYP3A5 polymorphism as intermediate metabolizers (1 allele with normal activity and 1 allele with little or no activity) and 7 patients were classified based poor metabolizers (2 alleles showing little or no activity) (See Figure below for sample test readout). All patients with intermediate CYP3A5 activity were found to have *1D|*3A, *1A|*3C, *1A|*3A, or *1D|*3C alleles. Patients with poor CYP3A5 activity were found to have *3A|*3A, *3C|*3C, or *3A|*3C. 3 intermediate metabolizers had acute cellular rejection and 1 intermediate metabolizer had mixed cellular/antibody mediated rejection within the first 3 months of transplant. 1 poor metabolizer had acute cellular rejection in the first 3 months of transplant. Of note, in addition to immunosuppression, 5 of the intermediate metabolizers and 2 of the poor metabolizers were found to have decreased metabolism of tramadol to the active form with increased risk of pharmacologic failure. Test results were available to the transplant pharmacists within 72 hours of ordering.
*Conclusions: A patient specific genomic test can be used in clinical practice to help guide medication therapy in kidney transplant recipients over and above measuring blood concentrations of immunosuppressants. Such an approach is actionable in the routine workflow of a transplant program and has potential to personalize pharmacotherapy beyond immunosuppression and minimize drug-drug interactions, reduce side effects, and therapeutic failure.
To cite this abstract in AMA style:
LeCorchick S, Lindberg L, Stapley W, Anand S, Morris D, Srinivas T. Clinical Utility of a Pharmacogenomic Testing Tool among Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-utility-of-a-pharmacogenomic-testing-tool-among-kidney-transplant-recipients/. Accessed January 28, 2026.« Back to 2019 American Transplant Congress