Performance Improvement Initiative: Ambulatory Tool to Improve Tacrolimus Intrapatient Variability (IPV) Monitoring in Kidney & Liver Transplant Recipients
1University of Cincinnati, Cincinnati, OH, 2UC Health, Cincinnati, OH
Meeting: 2019 American Transplant Congress
Abstract number: D14
Keywords: Immunosuppression, Kidney, Liver, Outcome
Session Information
Session Name: Poster Session D: Quality Assurance Process Improvement & Regulatory Issues
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: High tacrolimus (TAC) intrapatient variability (IPV) is associated with poorer outcomes. Manual monitoring of TAC IPV is labor intensive and not always feasible. As a performance improvement (PI) initiative, we developed and implemented an automated ambulatory longitudinal TAC IPV monitoring tool.
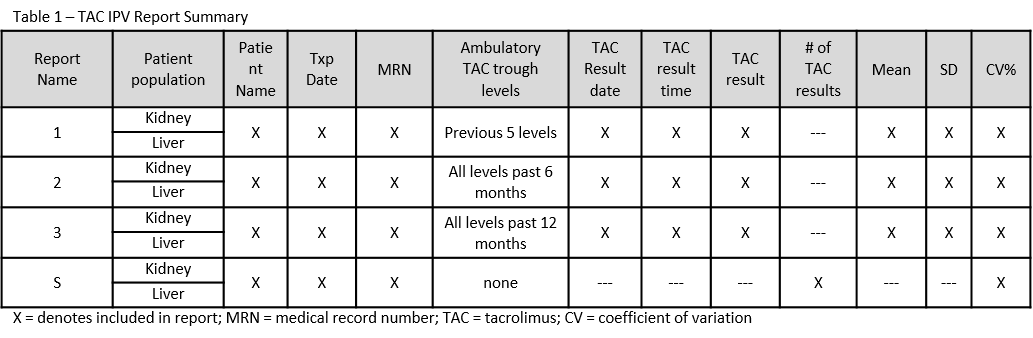
*Methods: We used a PLAN-DO-STUDY-ACT (PDSA) method to develop a screening report, “tool”. PLANNING steps identified and defined components of the TAC IPV report: patient population, TAC trough (type, quantity, date, time and result), TAC IPV calculation, report characteristics (fields, layout), and distribution time points. The DO steps involved report build with informational technologies team. Once built, the report was STUDIED through rounds of beta testing and ACTIONS for improvement were taken as necessary. The PDSA method continued to cycle until optimal TAC IPV reports were constructed. TAC IPV was defined as the coefficient of variation (CV% = standard deviation/mean) using the previous 5 TAC levels (report 1), TAC levels for the previous 6 (report 2) or 12 (report 3) months, using only ambulatory levels.
*Results: TAC IPV reports were created for kidney and liver recipients within 3 years of transplant (txp) and distributed to the txp team weekly for 6 months then monthly. Table 1 summarizes the reports. Beta testing identified multiple issues, such as: the exclusion of TAC troughs obtained within our own institution’s ambulatory laboratories (solution: revision of program rules to differentiate amongst institution locations) and data for repeat txp recipients merged together by name (solution: addition of txp date field). Additional testing revealed that reports containing 100 or more patients and 20-200 pages were time consuming and difficult to review. Subsequently, a summary TAC IPV (report S) was created with the same patients, the CV % and TAC trough level quantity which reduced the number of pages to 6-8 resulting in improved usability.
*Conclusions: This PI initiative led to the successful implementation of automated TAC IPV reports that are feasible, user friendly, with minimal cost. These will be used to characterize the natural evolution of TAC IPV post txp and to devise future initiatives aimed to identify txp recipient subgroups for targeted interventions. Utilizing automated TAC IPV monitoring could allow for the determination of TAC IPV thresholds for prospective intervention; thus minimizing long-term negative outcomes.
To cite this abstract in AMA style:
Kaiser TE, Ejaz NS, Tremblay S, Parrish NJ, Schinasi S, Harris J, Govil A, Anwar N, Alloway RR. Performance Improvement Initiative: Ambulatory Tool to Improve Tacrolimus Intrapatient Variability (IPV) Monitoring in Kidney & Liver Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/performance-improvement-initiative-ambulatory-tool-to-improve-tacrolimus-intrapatient-variability-ipv-monitoring-in-kidney-liver-transplant-recipients/. Accessed February 23, 2026.« Back to 2019 American Transplant Congress