A Prospective, Randomized, Multicenter Trial of Bela-Based CNI Free and Steroid Withdrawal Immunosuppression: Best Trial Two Year Characterization of Rejection and Renal Function
1University of Colorado School of Medicine, Denver, CO, 2University of Cincinnati, Cincinnati, OH, 3University of Wisconsin, Madison, WI, 4Tampa General, Tampa, FL, 5University of Minnesota, Minneapolis, MN, 6University of Illinois, Chicago, Chicago, IL
Meeting: 2019 American Transplant Congress
Abstract number: C174
Keywords: Induction therapy, Kidney transplantation, Rejection, Renal function
Session Information
Session Name: Poster Session C: Kidney: Acute Cellular Rejection
Session Type: Poster Session
Date: Monday, June 3, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
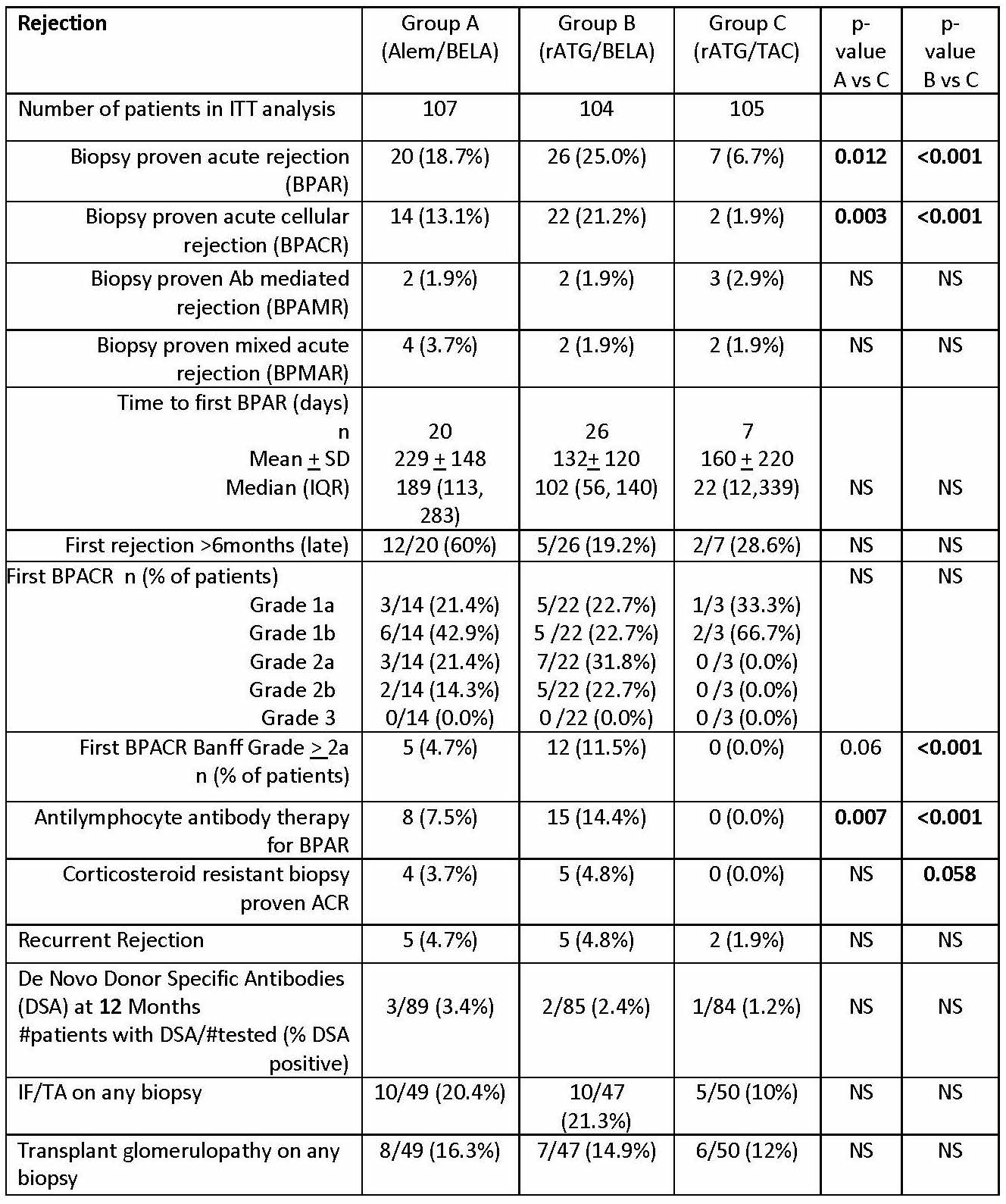
*Purpose: The BEST Trial (Belatacept-based Early Steroid Withdrawal Trial) was designed to compare two BELA-based early steroid withdrawal (ESW) regimens with a TAC-based ESW regimen at 2 years. Our hypothesis was that patients (pts) in BELA-based regimens will have higher acute rejection rates, but improved renal function.
*Methods: This longitudinal, multisite study was conducted under an FDA IND and IRB across 8 sites in adult kidney transplant pts. All pts received mycophenolate therapy and 5 days of steroids. Pts were randomized to 3 groups: alemtuzumab + BELA (Grp A), r-ATG + BELA (Grp B), and r-ATG + TAC (Grp C) and followed for 2 years.
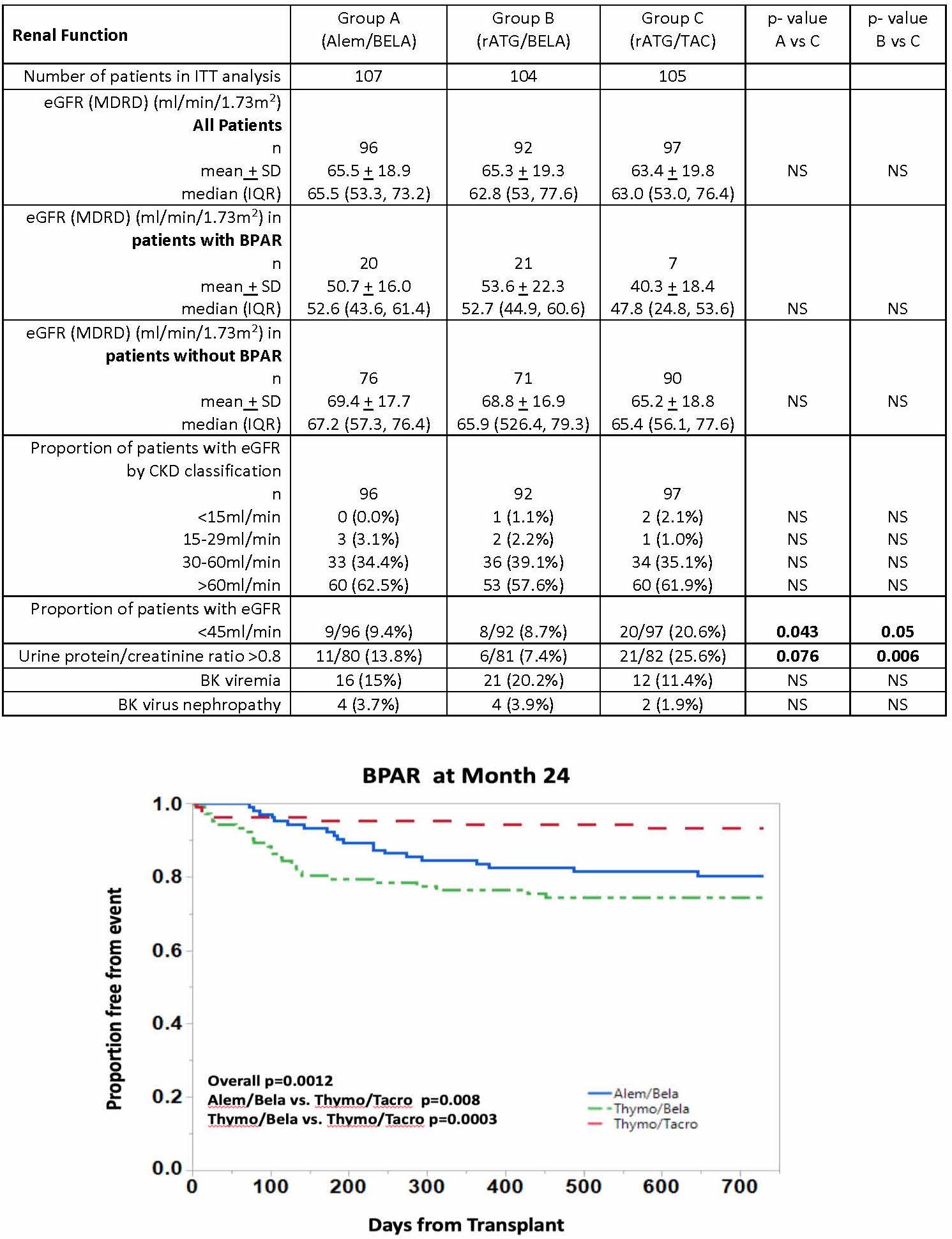
*Results: 316 pts were randomized. There were no statistical differences in baseline pt immunologic risk factors or donor characteristics. Rejection severity, as assessed by histology and requirement for rATG treatment, was higher in BELA groups (table, figure). Mean/median eGFR values were similar across groups in pts with and without rejection, however renal function was worse in pts with rejection, particularly in TAC group. While the proportion of pts in each group did not differ with respect to eGFR based on CKD classifications, significantly higher incidence of eGFR < 45 ml/min and proteinuria (UPC > 0.8) were present in the TAC group at 2 years.
*Conclusions: An increased incidence and severity of rejection was observed in BELA-based regimens compared to TAC, with an absolute increase in rejection incidence between BELA and CNI arms similar to those reported in BENEFIT. Rejection was associated with worse eGFR regardless of regimen, but was more pronounced in the TAC group. Despite lower rejection rates, the incidence of e-GFR<45ml/min and proteinuria > 0.8 g/g Cr was significantly greater in the TAC regimen at 2 years, suggesting potential longer-term renal function advantages over time with BELA therapy.
To cite this abstract in AMA style:
Wiseman A, Shields AR, Alloway RR, Kaufman D, Leone JP, Matas AJ, West-Thielke P, Lipscomb J, King E. A Prospective, Randomized, Multicenter Trial of Bela-Based CNI Free and Steroid Withdrawal Immunosuppression: Best Trial Two Year Characterization of Rejection and Renal Function [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/a-prospective-randomized-multicenter-trial-of-bela-based-cni-free-and-steroid-withdrawal-immunosuppression-best-trial-two-year-characterization-of-rejection-and-renal-function/. Accessed February 1, 2026.« Back to 2019 American Transplant Congress