A Novel Apparatus Mimicking Hypertensive Kidney With Primary Cultured Human Kidney Cells Using Rotational Force: Application To In Vitro Drug Screening In Kidney Transplant Recipients
1Internal Medicine, Seoul National University Hospital, Seoul, Korea, Republic of, 2Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea, Republic of, 3Internal Medicine, Hanyang University Medical Center, Seoul, Korea, Republic of, 4Internal Medicine, Cheju Halla General Hospital, Cheju, Korea, Republic of, 5Kidney Research Institute, Seoul National University Hospital, Seoul, Korea, Republic of
Meeting: 2019 American Transplant Congress
Abstract number: C12
Keywords: Drug interaction, Fibrosis, Immunosuppression
Session Information
Session Name: Poster Session C: Immunosuppression Preclinical Studies
Session Type: Poster Session
Date: Monday, June 3, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
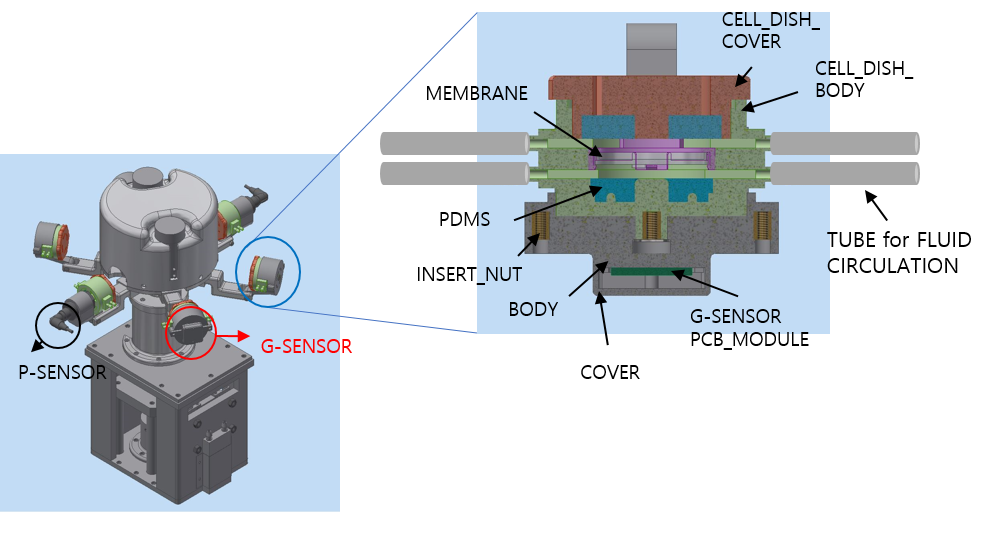
*Purpose: Cell culture is an essential part of the development for various medical technologies such as identifying the cause of the disease and developing new medicines. And high blood pressure is a leading cause of various kidney disease and eventually results in kidney fibrosis and end stage renal disease. We successfully developed a novel cell culture device with primary cultured kidney cells such as glomerular endothelial cells, podocytes, mesangial cells, and proximal tubular cells. Afterwards we applied rotational force to mimic hypertensive environment of the kidney disease. The cell culture device is also considered essential for in-vitro experiments for drug screening in various disease modeling such as kidney transplantation.
*Methods: The study is about a cell culture device that can be mimicked biological pressure and shear stress environments. Four types of human kidney cells were extracted from glomeruli and cultured using the system under different pressure levels, 4mmHg and 8 mmHg, and exposure times, 3 hours, 48 hours, and 72 hours. The pressure can be applied continuously using centrifugal force by rotating the cell culture dish, and the pressure can be controlled by changing the number of rotations. This device can apply the shear stress to cells by circulating the culture media using the pump. Two different peristaltic pumps are equipped to regulate the flow independently. The device can be mounted with different kinds of cell culture dish trays, so it can also be use commercial dish such as 100 pie dish and 6 well dish and multi-cell culture is also available with customized dish. Angiotensin receptor blocker (ARB), an anti-hypertensive drug was treated to evaluate the effect to pressure-induced fibrosis.
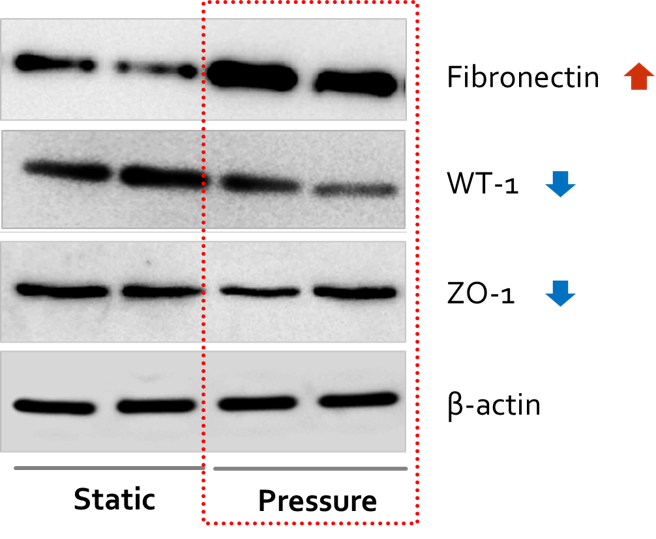
*Results: We observed that the viability of primary cultured kidney cells drops as the pressure increases up to 10 mmHg. Afterwards we confirmed that the expression of fibronectin was increased and WT-1, ZO-1 was decreased under 4 mmHg of pressure after 48 hours (figure 1). Also, mRNA expressions of podocalyxin, synaptopodin, WT-1, and KLF15 was decreased. This suggests that fibrosis was properly induced after stimulating primary cultured cell with rotational pressure. After treating ARB to these cells, the protein expression of αSMA increased and KLF15 showed significant decrease, which implicates that pressure-induced fibrosis has been improved with ARB treatment. Given these results, we could evaluated the efficacy or toxicity of other drugs such as immunosuppressants.
*Conclusions: This device is a new concept cell culture device that can apply continuous pressure and shear stress to cells using simple principles. Furthermore immunosuppressant drug screening can be done using patient-derived primary cultured kidney cells.
To cite this abstract in AMA style:
Kim Y, Lee S, Moon J, Kim J, Yu M, Lee C, Kim Y, Yang S, Jeong J, Seon J. A Novel Apparatus Mimicking Hypertensive Kidney With Primary Cultured Human Kidney Cells Using Rotational Force: Application To In Vitro Drug Screening In Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/a-novel-apparatus-mimicking-hypertensive-kidney-with-primary-cultured-human-kidney-cells-using-rotational-force-application-to-in-vitro-drug-screening-in-kidney-transplant-recipients/. Accessed January 22, 2026.« Back to 2019 American Transplant Congress