Pharmacokinetics of Innovative versus Generic Valganciclovir in Kidney Transplant Recipients
1Nephrology and Mineral Metabolism, Instituto Nacional de Ciencias Médicas y Nutrición, Ciudad de Mexico, Mexico, 2Transplant, Instituto Nacional de Ciencias Médicas y Nutrición, Ciudad de Mexico, Mexico, 3Pharmacology, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Ciudad de Mexico, Mexico
Meeting: 2019 American Transplant Congress
Abstract number: B239
Keywords: Cytomeglovirus, Kidney transplantation, Pharmacokinetics, Prophylaxis
Session Information
Session Name: Poster Session B: Kidney Infections
Session Type: Poster Session
Date: Sunday, June 2, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: In kidney transplant recipients (KTR), cytomegalovirus (CMV) is a common viral infection associated with significant morbidity and mortality. In the absence of prophylaxis, CMV disease in high-risk recipients (D+/R-) has an incidence of 60%. Valganciclovir (VGCV) is commonly used for CMV prophylaxis. Generic VCGV is likely as effective as innovative VGCV and its use could be a safe strategy to reduce costs.Our aim was to compare pharmacokinetic parameters of innovative versus generic VGCV in KTR undergoing CMV prophylaxis with VGCV in the early post-transplant period.
*Methods: Crossover pharmacokinetic study in stable adult KTR undergoing prophylaxis with VGCV (900 mg daily), between days 31-90 post-transplant. All patients received both formulations (innovative and generic). At steady state (after 3 days), ganciclovir (GCV) blood concentration were measured at different times (0,0.5,1,2,4,8,12 and 24 h) and were determined by high performance liquid chromatography.
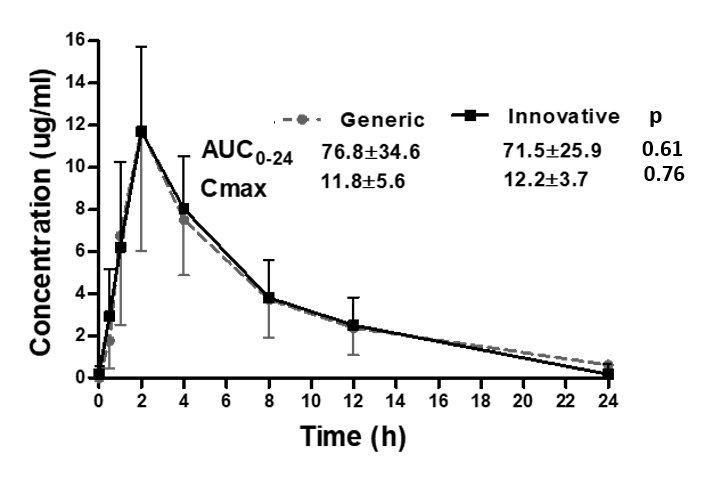
*Results: Eight adult KTR were included, age was 35.6 ± 9.8 years, 87.5% were males, body weight was 67.4 ± 13.7 kg, BSA was 1.7 ± 0.1m2, all received their first kidney-transplant, 62.5% were deceased donor recipients, eGFR using CKD-EPI was 82.2 ±20.3 ml/min/m2, 75% had (D+/R+) CMV serostatus, induction therapy with thymoglobulin was used in 75% of patients, all recipients were under maintenance immunosuppression with tacrolimus, MMF and steroids. A total of 64 samples were available for determining GCV plasma concentration (eight samples per patient). Pharmacokinetics was similar with both formulations for innovative and generic VGCV, figure.
*Conclusions: Pharmacokinetic studies in the early post-transplant period are needed to asses if bioavailability between innovative and generic formulation is similar, and concomitant drugs or unstable renal function can change drug plasma concentration. In the present study, both VGCV formulations provide similar GCV plasma concentration. Our study is limited by comparing only one generic formulation, and we cannot conclude that all generic VGCV formulations would behave similarly.
To cite this abstract in AMA style:
Parra-Avila I, Cohen-Bucay A, Alberu J, Castañeda-Hernandez G, Morales-Buenrostro LE. Pharmacokinetics of Innovative versus Generic Valganciclovir in Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/pharmacokinetics-of-innovative-versus-generic-valganciclovir-in-kidney-transplant-recipients/. Accessed January 31, 2026.« Back to 2019 American Transplant Congress