Comparing the Rate of Opportunistic Infection after Renal Transplant between Alemtuzumab and Basiliximab for Induction Immunosuppression
1College of Medicine, University of Nebraska Medical Center, Omaha, NE, 2Internal Medicine Division of Nephrology, University of Nebraska Medical Center, Omaha, NE, 3Biostatistics, University of Nebraska Medical Center, Omaha, NE, 4Donate Life, Nebraska Medicine, Omaha, NE, 5Internal Medicine Division of Infectious Disease, University of Nebraska Medical Center, Omaha, NE
Meeting: 2019 American Transplant Congress
Abstract number: B206
Keywords: Cytomeglovirus, Infection, Kidney/liver transplantation, Simulect
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Sunday, June 2, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
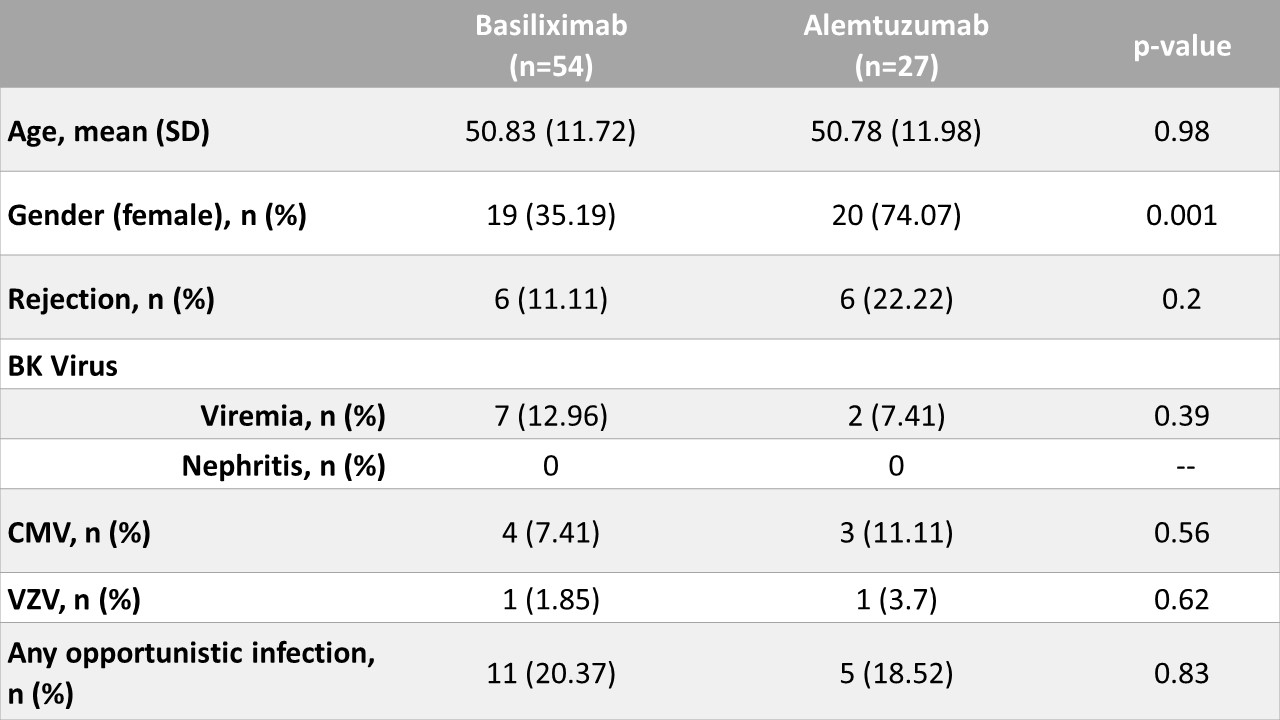
*Purpose: The aim of our study was to compare the outcomes one-year after renal transplant with a focus on opportunistic infections in patients who received either alemtuzumab or basiliximab for induction immunosuppression therapy.
*Methods: We included all patients who received induction with alemtuzumab for renal transplant between 2011 and 2016 at our center. These were matched 1:2 to basiliximab recipients by age and date of transplant. Electronic medical records were searched for evidence of the following opportunistic infections; BK virus, CMV, VZV, HSV, Cryptococcus, and Histoplasma. Continuous variables were compared using t-tests or Wilcoxon rank-sum tests and categorical variables using Fisher’s tests or chi-square tests. Binary outcomes were compared using Cochran-Mantel-Haenszel tests. Time-to-event outcomes were plotted using the Kaplan-Meier method and compared between groups using log-rank tests.
*Results: Most patients were maintained on tacrolimus, mycophenolate, and prednisone. No statistically significant differences in the following outcomes were detected: delayed graft function (p = 0.76), graft loss (p = 0.99), or rejection (p = 0.2). Opportunistic infection rates were not significantly different at 1-year post-transplant (p = 0.83). Time-to-infection (p = 0.84) and time-to-death (p=0.21) were similar in both groups, but time-to-rejection was longer in the basiliximab group vs alemtuzumab group (p = 0.04).
*Conclusions: Despite a difference in immunological risk between the 2 groups, we did not detect any significant differences in outcomes at 1-year post-transplantation between higher immunological risk patients receiving alemtuzumab and lower risk patients receiving basiliximab. Early opportunistic infectious rates were similar. Surprisingly, no fungal infections were detected at 1-year post-transplant which may represent a delay in detection of any potential risk differences.
To cite this abstract in AMA style:
Seaman JA, Westphal SG, Qiu F, Bremers D, Florescu D. Comparing the Rate of Opportunistic Infection after Renal Transplant between Alemtuzumab and Basiliximab for Induction Immunosuppression [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/comparing-the-rate-of-opportunistic-infection-after-renal-transplant-between-alemtuzumab-and-basiliximab-for-induction-immunosuppression/. Accessed February 18, 2026.« Back to 2019 American Transplant Congress