Vaccination And Allosensitization In Solid Organ Transplant Candidates
1Pharmacy, University of Chicago Medicine, Chicago, IL, 2Pharmacy, Barnes-Jewish Hospital, St. Louis, MO, 3Pharmacy, Tampa General Hospital, Tampa, FL, 4Internal Medicine, The Johns Hopkins School of Medicine, Baltimore, MD, 5Heart Failure and Heart Transplantation, Washington University School of Medicine, St. Louis, MO, 6Pulmonary and Critical Care Medicine, Washington University School of Medicine, St. Louis, MO
Meeting: 2019 American Transplant Congress
Abstract number: A368
Keywords: Alloantibodies, Highly-sensitized, HLA antibodies, Waiting lists
Session Information
Session Name: Poster Session A: Transplant Infectious Diseases
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: Clinical practice guidelines recommend vaccinating patients awaiting solid organ transplantation (SOT) against vaccine-preventable infections, but there is little evidence to suggest that vaccination reduces the incidence or severity of post-transplant infection; this calls into question the magnitude of benefit derived from vaccinating patients on the SOT waiting list. Furthermore, emerging data suggest that vaccination may increase the risk of allosensitization.
*Methods: This was a single-center, retrospective cohort study at Washington University and Barnes-Jewish Hospital, St. Louis, MO. We evaluated 167 SOT candidates to assess the development of new human leukocyte antigen (HLA) antibodies after vaccination. All adult (age ≥ 18 years) heart, kidney, lung, pancreas or multi-organ transplant candidates listed for transplantation at Barnes-Jewish Hospital between January 1, 2006 to December 31, 2013 who received at least one documented vaccination (hepatitis A, hepatitis B, influenza, pneumococcal, tetanus, and/or varicella) were eligible for this study.
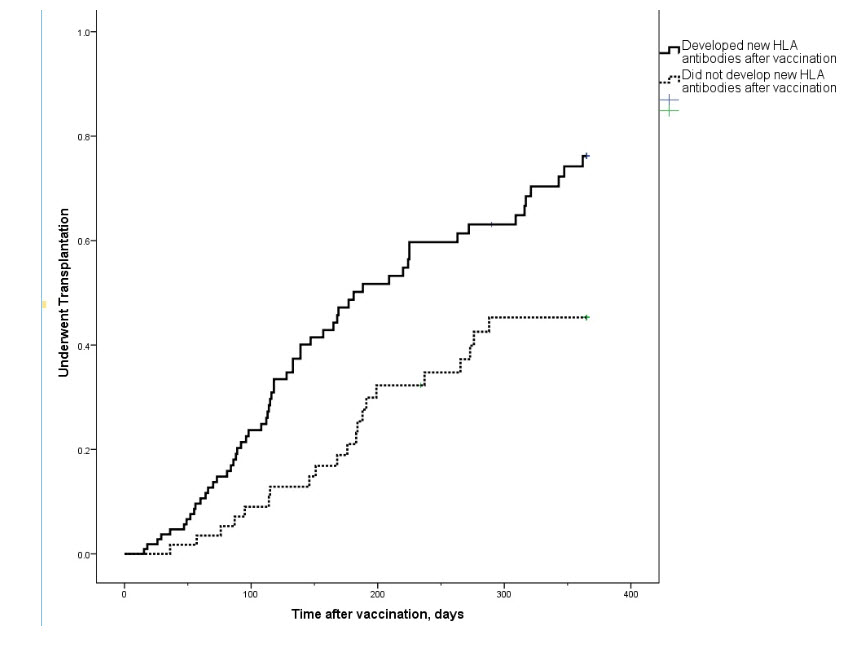
*Results: Overall, 58 patients (35%) developed new HLA antibodies following vaccination. Only 20 (34%) of those cleared the new antibodies, a median of 137 days later. Patients who developed new antibodies after vaccination were significantly less likely to undergo transplantation than those who did not develop new antibodies (36% versus 51%, p = 0.036), Figure 1. Patients who received multi-vaccine regimens including an influenza vaccine had a higher incidence of post-vaccination allosensitization than those that did not include an influenza vaccine (47% versus 21%, p = 0.041). Multivariate analyses demonstrated that patients who were allosensitized prior to vaccination were significantly more likely to develop new antibodies post-vaccination (adjusted odds ratio 2.44, 95% CI 1.26-4.73, p = 0.008).
*Conclusions: This study suggests that the risk of vaccination, especially in those who are allosensitized, may be greater than the benefit of vaccination. Although our data demonstrate that vaccination is associated with allosensitization, we would not necessarily advocate withholding vaccination in patients awaiting transplantation. There is equipoise to conduct randomized controlled trials examining the risks and benefits of vaccination in patients awaiting transplantation.
To cite this abstract in AMA style:
Lourenco L, Bain KB, Bowman L, Brennan DC, Ewald GA, Hachem RR. Vaccination And Allosensitization In Solid Organ Transplant Candidates [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/vaccination-and-allosensitization-in-solid-organ-transplant-candidates/. Accessed February 9, 2026.« Back to 2019 American Transplant Congress