Comparison of Initial Sirolimus (SRL) and Everolimus (EVR) Exposure Patterns in Kidney Transplant Recipients
Hospital do Rim, Nephrology Division, EPM - UNIFESP, São Paulo, Brazil
Meeting: 2019 American Transplant Congress
Abstract number: A273
Keywords: Kidney transplantation, Pharmacokinetics, Sirolimus (SLR)
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: Despite distinct pharmacokinetic properties, SRL and EVR bind to the same intracellular target protein, suggesting that equivalent blood concentrations may exert similar pharmacodynamic effect.
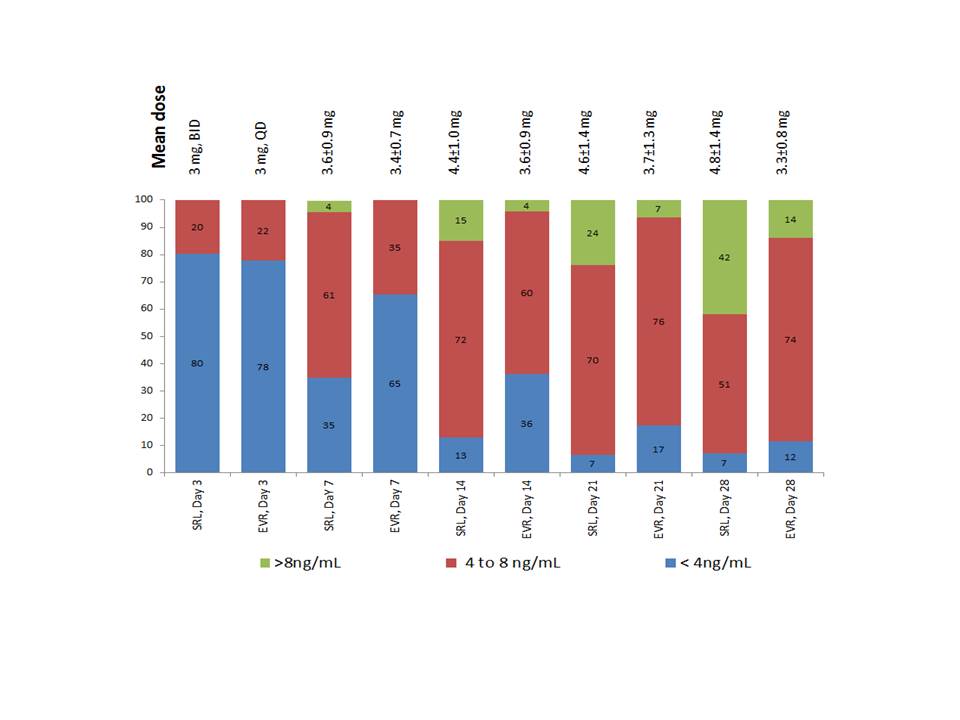
*Methods: Methods: This large, single center, prospective randomized trial compares the efficacy, safety, therapeutic drug monitoring and an array of candidate biomarkers of the use of SRL (3 mg QD adjusted to maintain concentrations between 4 to 8 ng/mL), EVR (3 mg BID adjusted to maintain concentrations between 4 to 8ng/mL), or MPA (720 mg BID) in kidney transplant recipients receiving 3 mg/kg r-ATG induction therapy, tacrolimus and faster prednisone taper (Clinicaltrials.govNCT03468478). Our hypothesis is that similar initial 3 mg doses of SRL (QD) and EVR (BID) would provide comparable blood concentrations and similar responses to dose adjustments, despite the differences in pharmacokinetic profiles. Methods: This preliminary analysis included 153 adult recipients of first kidney transplants with at least one month of follow up. First dose adjustments consisted of a 30% increase in drug dose for all patients with blood concentrations below 4 ng/ml. Subsequent dose adjustments were made twice a week to achieve blood concentrations of 6 ng/ml.
*Results: There were no differences in demographic characteristics of the study population. At day 3 mean trough concentrations of SRL and EVR were comparable (3.2±1.3 vs. 3.8±0.9 ng/mL, p=0.084) although a low percentage of patients showed concentration above 4 ng/ml (20% vs. 22%, respectively). From day 3 to day 28 a higher proportion of patients receiving SRL reached target therapeutic concentration compared to EVR (Figure). Compared to day 3, at day 28 SRL doses were 20% higher (3.3±0.8 mg/day) while EVR dose were 60% higher (4.8±1.4 mg/day), producing mean trough concentrations of (8.0±4.0 and 6.3±1.7 ng/mL, p= 0.023), respectively. Higher increases in dose-corrected concentrations from day 1 to 28 were observed with SRL (1.3±0.5 to 2.6±1.5 ng/mL/mg) compared to EVR (1.2±0.4 to 1.3±0.5).
*Conclusions: Despite using similar initial doses and therapeutic monitoring strategies, a higher proportion of patients receiving SRL reach therapeutic drug concentrations faster with lower doses compared to those receiving EVR.
To cite this abstract in AMA style:
Felipe CRosso, Viana L, Cristelli MPontello, Nakamura M, Ramagnoli L, Braga S, Lima V, Carnevalle A, Casarini D, Pestana JMedina, Junior HTedescoSilva. Comparison of Initial Sirolimus (SRL) and Everolimus (EVR) Exposure Patterns in Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/comparison-of-initial-sirolimus-srl-and-everolimus-evr-exposure-patterns-in-kidney-transplant-recipients/. Accessed February 18, 2026.« Back to 2019 American Transplant Congress