The Effect of a Novel Dosing Protocol for the De Novo Use of LCP Tacrolimus in Kidney Transplant Recipients Managed on a Steroid-Free Maintenance Immunosuppression Regimen
1School of Pharmacy, MHealth U of MN Medical Center-Fairview, Mpls, MN, 2Medicine, MHealth U of MN Medical Center-Fairview, Mpls, MN, 3Surgery, U of Pennsylvania, Phila, PA
Meeting: 2019 American Transplant Congress
Abstract number: A241
Keywords: Efficacy, Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: It is not known if an extended release formulation of tacrolimus (LCPT, Envarsus XR®) can be successfully dosed to achieve an early therapeutic trough (TTR) >8mcg/L, which may be important for patients on a steroid-free regimen. Herein we evaluated the TTR and safety data of a novel, de novo LCPT dosing protocol in kidney transplant recipients managed without corticosteroids.
*Methods: This prospective observational cohort study included adult kidney recipients who received thymoglobulin induction and a steroid-free maintenance regimen consisting of mycophenolate plus immediate release tacrolimus (IRT) vs LCPT. Exclusion criteria were prior or simultaneous extra-renal organ transplant, participation in another immunosuppression study, or change in regimen before POD30. The primary endpoint was the achievement of TTR on post-operative day (POD) 6, 10, 14, 21, and 28. Secondary endpoints included delayed graft function (DGF), hyperkalemia, rejection, infection, and adverse drug reactions (ADR) through POD90.
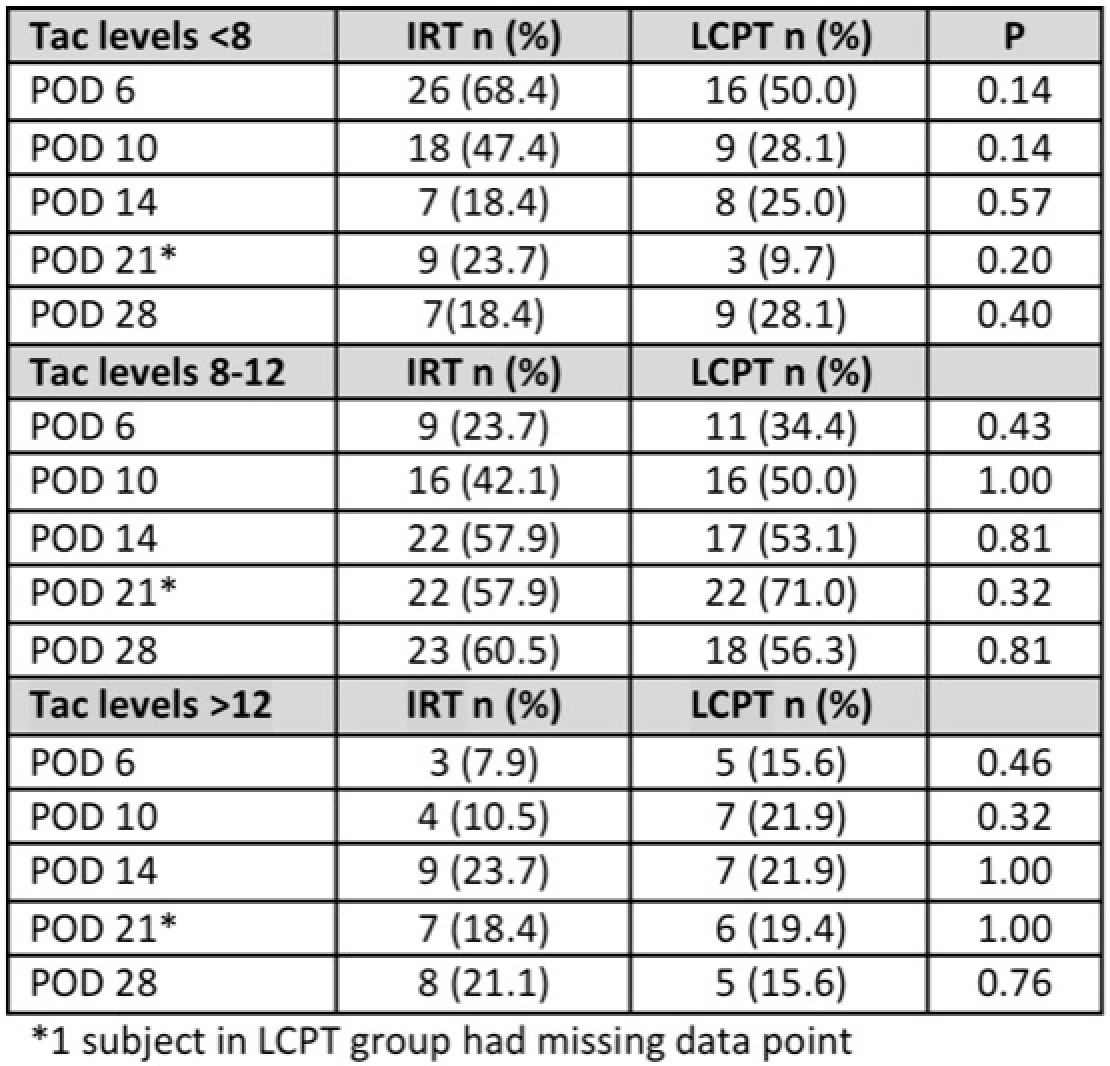
*Results: Compared to the IRT group (N=38), the LCPT group (N=32) was older (52.7 vs 60.1 years, p=0.09) and had a higher mean KDPI (47.4 vs 69.2, p=0.02), with otherwise similar characteristics. POD6 mean tacrolimus trough level was 6.3 mcg/L for IRT vs 8.6 mcg/L for LCPT, p=0.01. The proportion of patients in each group with levels <8, 8-12, or >12 mcg/L was similar at all time points, with a trend in LCPT group to have more patients >8 mcg/L at POD6 and POD10 (Figure 1). There were no significant differences between groups for secondary outcomes. While not statistically significant, DGF was less in the LCPT group (9.4% vs 29% for IRT, p=0.07) and hyperkalemia in the LCPT group was 9.4% vs 21.0% for IRT (p=0.67).
*Conclusions: This novel LCPT de novo dosing protocol resulted in a TTR by POD6, with trough levels similar to IRT at all other measured time points. Achieving a TTR early after transplant may be important for patients managed on a corticosteroid-free regimen, or for those at increased immunologic risk, but must be balanced against toxicities. The trends in decreased DGF and hyperkalemia in the LCPT group may be advantageous and warrants further study in a larger population.
To cite this abstract in AMA style:
Wolff R, Nasstrom A, Borscheid C, Martin C, Temelie A, Spong R, Keys D, Dunn TB. The Effect of a Novel Dosing Protocol for the De Novo Use of LCP Tacrolimus in Kidney Transplant Recipients Managed on a Steroid-Free Maintenance Immunosuppression Regimen [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/the-effect-of-a-novel-dosing-protocol-for-the-de-novo-use-of-lcp-tacrolimus-in-kidney-transplant-recipients-managed-on-a-steroid-free-maintenance-immunosuppression-regimen/. Accessed February 15, 2026.« Back to 2019 American Transplant Congress