Evidence to Support the Use of Kidney Allograft Function as a Clinical Trial Outcome Measure
Nephrology, University of British Columbia, Vancouver, BC, Canada
Meeting: 2019 American Transplant Congress
Abstract number: A177
Keywords: Glomerular filtration rate (GFR), Kidney transplantation, Outcome
Session Information
Session Name: Poster Session A: Biomarkers, Immune Monitoring and Outcomes
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: As a biomarker, kidney allograft function has been rejected as clinical trial outcome measure because it is not predictive of allograft survival. In this analysis we determined the utility of eGFR as potential clinical reported outcome (ClinRo) (i.e. a measure of how a patient lives, feels or functions in daily life) by determining the association of eGFR with patient hospitalization.
*Methods: Using the United States Renal Data System, we calculated the eGFR at 12 months after transplantation in a cohort of Medicare insured adults with a functioning kidney transplant between January 1, 2000 and December 31, 2013. We determined the association between the probability of hospitalization and the number of days spent in hospital in the year following the GFR measurement (12-24 months post-transplant). We then constructed multivariable regression models of the odds of hospitalization and risk of additional days in hospital as a function of eGFR.
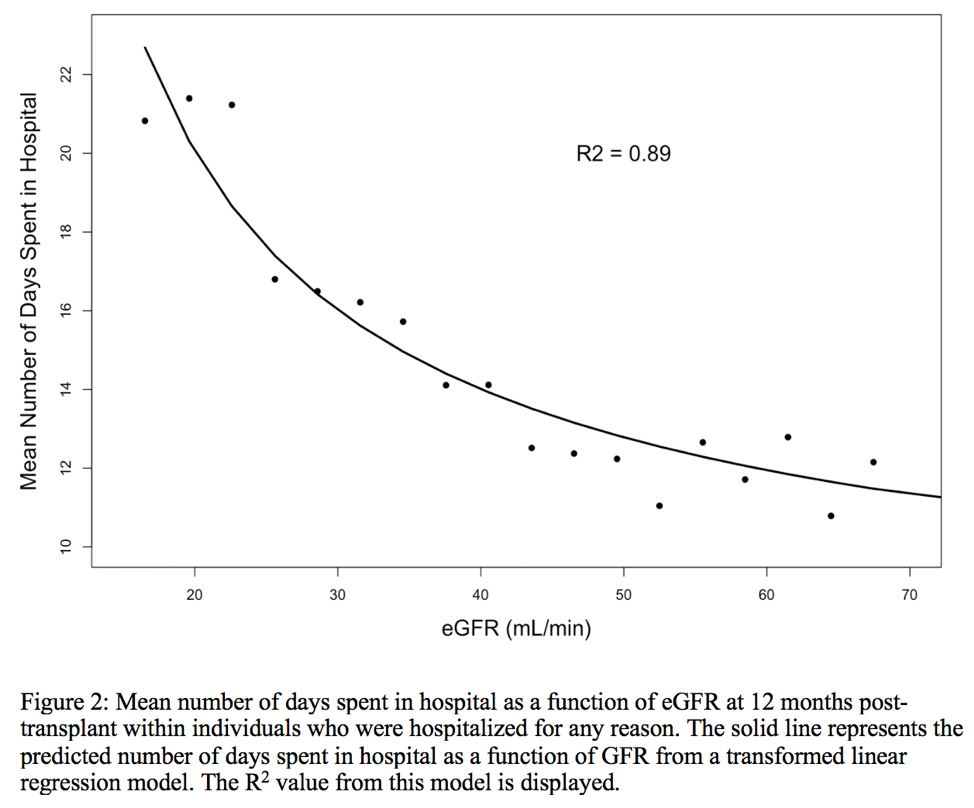
*Results: In the final analytic cohort (n=95,500), the unadjusted probability of hospitalization and the mean number of days spent in hospital between 12 and 24 months post-transplant as a function of hospitalization is shown in Figure 1 and 2, respectively. There is a strong association between the probability of hospitalization regardless of the type of donor and, and also the predicted number of days in hospital. Results were consistent in multivariable regression models.
*Conclusions: eGFR at 12 months post-transplant is strongly associated the likelihood of future hospitalizations in univariable and multivariable models. This study advances evidence that eGFR at 12 months post-transplant may be reconsidered as a clinical trial outcome under the FDA category of Clinician-reported outcomes (Clin-Ro).
To cite this abstract in AMA style:
Kadatz M, Gill J, Clark S, Gill JS. Evidence to Support the Use of Kidney Allograft Function as a Clinical Trial Outcome Measure [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/evidence-to-support-the-use-of-kidney-allograft-function-as-a-clinical-trial-outcome-measure/. Accessed February 15, 2026.« Back to 2019 American Transplant Congress