Infectious Complications in Tocilizumab-Treated Kidney Transplant Recipients
Cedars Sinai Medical Center, Los Angeles, CA
Meeting: 2019 American Transplant Congress
Abstract number: 465
Keywords: Adverse effects, Immunosuppression, Infection, Kidney
Session Information
Session Name: Concurrent Session: Novel Insights in Kidney Infections
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:42pm-3:54pm
Presentation Time: 3:42pm-3:54pm
Location: Room 311
*Purpose: Tocilizumab blocks IL-6 signaling, inhibiting maturation of B cells to plasma cells and antibody production. We have used tocilizumab since 2012 to treat kidney transplant recipients with donor specific antibodies (DSA) and/or antibody-mediated rejection (ABMR). Herein, we describe infectious complications among tocilizumab-treated patients.
*Methods: Medical records of 66 kidney transplant recipients treated with tocilizumab 8 mg/kg IV monthly for DSA +/- ABMR at our center from April 2012 to November 2018 were reviewed to identify any infection while being treated with tocilizumab and up to one year after cessation of therapy. Time to first infectious episode was calculated using the Kaplan-Meier product limit method and predictors of infection were assessed with Cox proportional hazards models.
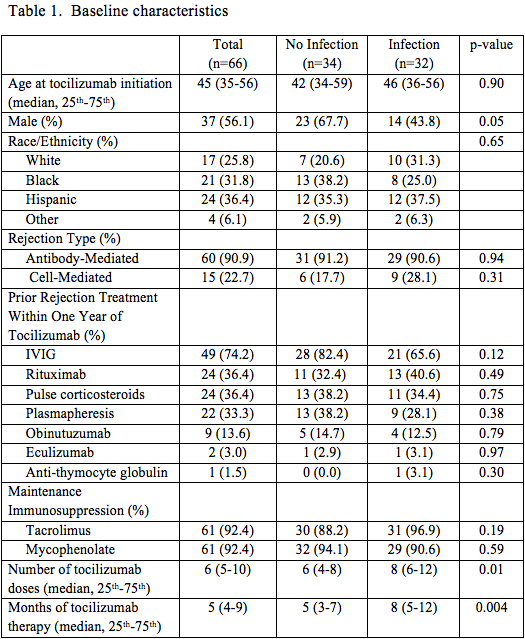
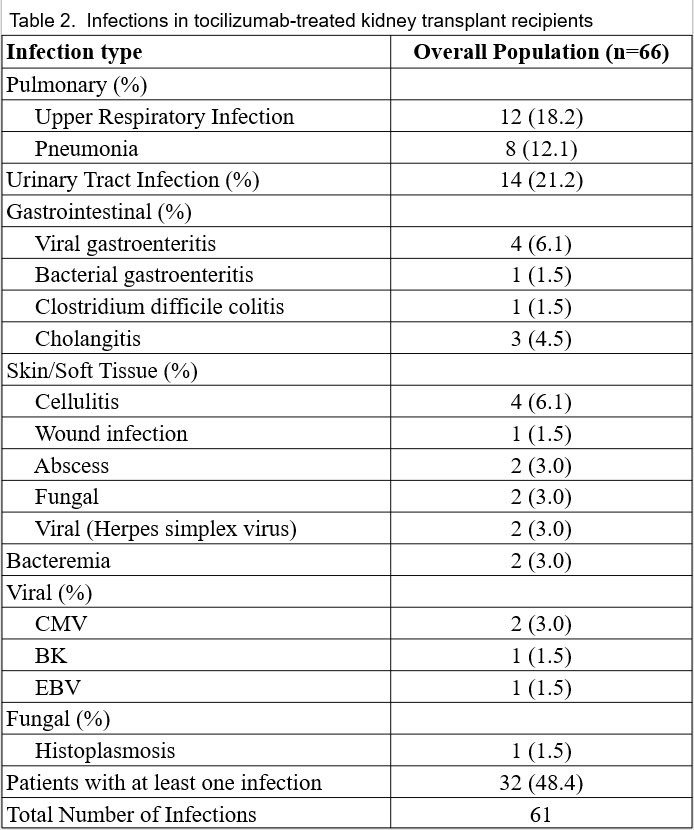
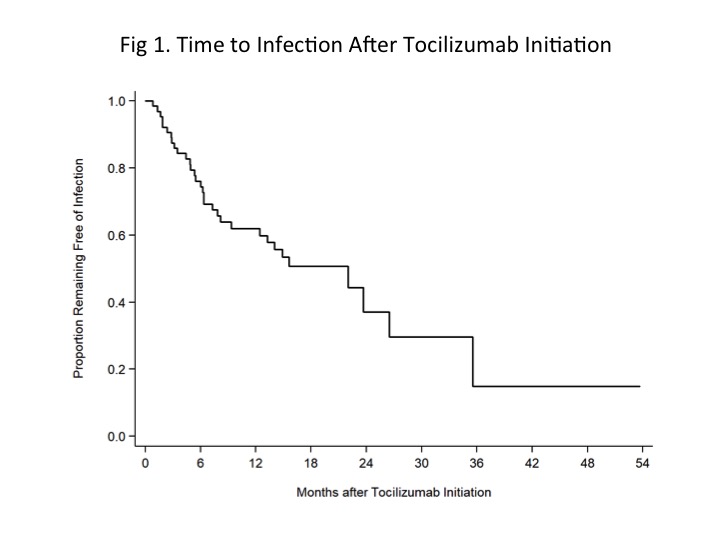
*Results: A median of 6 doses (IQR: 5-10) of tocilizumab were administered to 66 kidney recipients over 64 person-years of follow-up. Thirty-two (48%) patients developed 61 infections (incidence rate: 95 infections/100 person-years). There were a total of 28 hospitalizations involving 18 patients, but no infection-related deaths. Characteristics (table 1) were similar between recipients with and without infections aside from duration of tocilizumab treatment, which was longer among recipients with infections. Most patients (n=56, 85%) received additional therapies for DSA/ABMR within 1 year of tocilizumab initiation. Urinary tract and upper respiratory infections were the most common whereas viral and fungal infections were rare (table 2). The Kaplan-Meier infection-free survivor function was 60% at 12 months and 37% at 24 months (fig 1). On univariate analysis, no specific factor was associated with incidence of infection.
*Conclusions: Infections were common among tocilizumab-treated kidney recipients, although most were minor and readily-treated.
To cite this abstract in AMA style:
Sethi S, Peng A, Najjar R, Vo A, Jordan S, Huang E. Infectious Complications in Tocilizumab-Treated Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/infectious-complications-in-tocilizumab-treated-kidney-transplant-recipients/. Accessed January 29, 2026.« Back to 2019 American Transplant Congress