Evaluation of Influenza Virus Antibody Response in Naturally Infected and Vaccinated Transplant Recipients by Protein Microarray
University Health Network, Toronto, ON, Canada
Meeting: 2019 American Transplant Congress
Abstract number: 173
Keywords: Antibodies, Infection, Vaccination
Session Information
Session Name: Concurrent Session: Vaccines and Viruses
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Room 313
*Purpose: Influenza A virus (IAV) infection is known to be an important cause of morbidity in solid organ transplant (SOT) recipients. The objective of our study was to analyze the detailed repertoire of antibody production in SOT patients after influenza infection or vaccination, using a microarray technique that simultaneously quantifies antibodies against multiple influenza antigens.
*Methods: Two groups of adult SOT recipients were included in this study: 1) patients with IAV infection and 2) patients who were vaccinated with standard dose trivalent seasonal influenza vaccine. Serum was either collected at the time of IAV infection diagnosis and four weeks after or immediately prior to vaccination and four weeks later. A diverse collection of influenza antigens (n=86), including those from various subtypes, host origins and geographical locations, were printed onto nitrocellulose-coated slides and probed using patient sera and fluorescently-conjugated IgG and IgA secondary antibodies. Following measurement of median fluorescent intensity (MFI), antibody responses (fold changes in MFI) were compared among naturally infected and vaccinated patients, using significance analysis of microarrays (SAM) with a false discovery rate <1%.
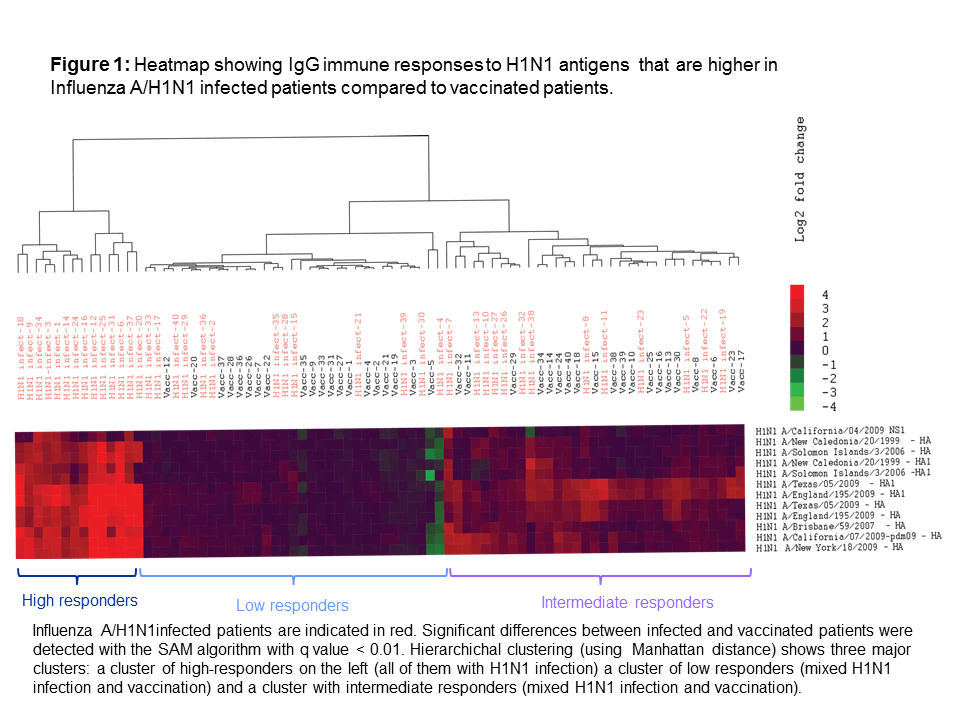
*Results: A total of 120 patients were included: 80 in the IAV infection group (40 A/H1N1, 40 A/H3N2) and 40 in the vaccine group. The median time from transplant to infection/vaccination was 3.0 (IQR 0.8-8-1) years. All IAV infected patients were treated with oseltamivir. H1N1 infected patients showed a significantly higher IgG and IgA antibody response for 12 out of 26 H1N1 antigens (11 out of 12 targeting hemagglutinins) compared to vaccine patients (Figure 1).
For the remaining 14 antigens, vaccine responses were similar to those with H1N1 infection. With regard to the IgG and IgA immune response against H3N2 antigens, H3N2 infected patients produced significantly stronger immune responses relative to vaccine patients for 17 out of 35 antigens. Antibody response between infected and vaccinated patients was similar for the remaining 18 antigens. IAV infection (H1N1 and H3N2 pooled) resulted in stronger immune responses relative to vaccination for heterotypic H5N1 antigens (IgG: 4/6 antigens, IgA: 1/6 antigens).
*Conclusions: This study provides novel data on humoral immune response after IAV infection and influenza vaccination. Natural IAV infection uniformly results in more pronounced and diverse humoral immune responses against IAV antigens in SOT recipients compared to vaccination.
To cite this abstract in AMA style:
Hirzel C, Chruscinski A, Ferreira VH, L'Huillier AG, Humar A, Kumar D. Evaluation of Influenza Virus Antibody Response in Naturally Infected and Vaccinated Transplant Recipients by Protein Microarray [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-influenza-virus-antibody-response-in-naturally-infected-and-vaccinated-transplant-recipients-by-protein-microarray/. Accessed February 26, 2026.« Back to 2019 American Transplant Congress