Effect of Everolimus with Reduced-Exposure Tacrolimus and Early Steroid Elimination in Pediatric Kidney Transplant Recipients: 36-Month Outcomes from the CRADLE Study
1A2314 Study Group, Basel, Switzerland, 2Novartis Pharma AG, Basel, Switzerland
Meeting: 2019 American Transplant Congress
Abstract number: 135
Keywords: Efficacy, Kidney transplantation, Renal function, Safety
Session Information
Session Name: Concurrent Session: Kidney: Pediatrics I
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:30pm-5:42pm
Presentation Time: 5:30pm-5:42pm
Location: Room 304
*Purpose: To compare 36-month (M) efficacy and safety outcomes of an everolimus+reduced-exposure tacrolimus (EVR+rTAC)+steroids (CS) withdrawal regimen vs mycophenolate mofetil+standard-exposure TAC (MMF+sTAC)+CS in pediatric kidney transplant recipients (pKTxRs) from the CRADLE study (NCT01544491).
*Methods: In this 12M core+24M follow-up study, 106 pKTxRs (≥1-<18 years) were randomized (1:1) at 4-6 weeks post transplantation (Tx) to either switch to EVR+rTAC (N=52) with CS withdrawal (at 6M post-Tx) or continue MMF+sTAC (N=54) regimen. Assessments included the incidence of composite efficacy failure (CEF) of BPAR, graft loss, or death and its individual components, eGFR (abbreviated Schwartz formula), and safety outcomes at M36.
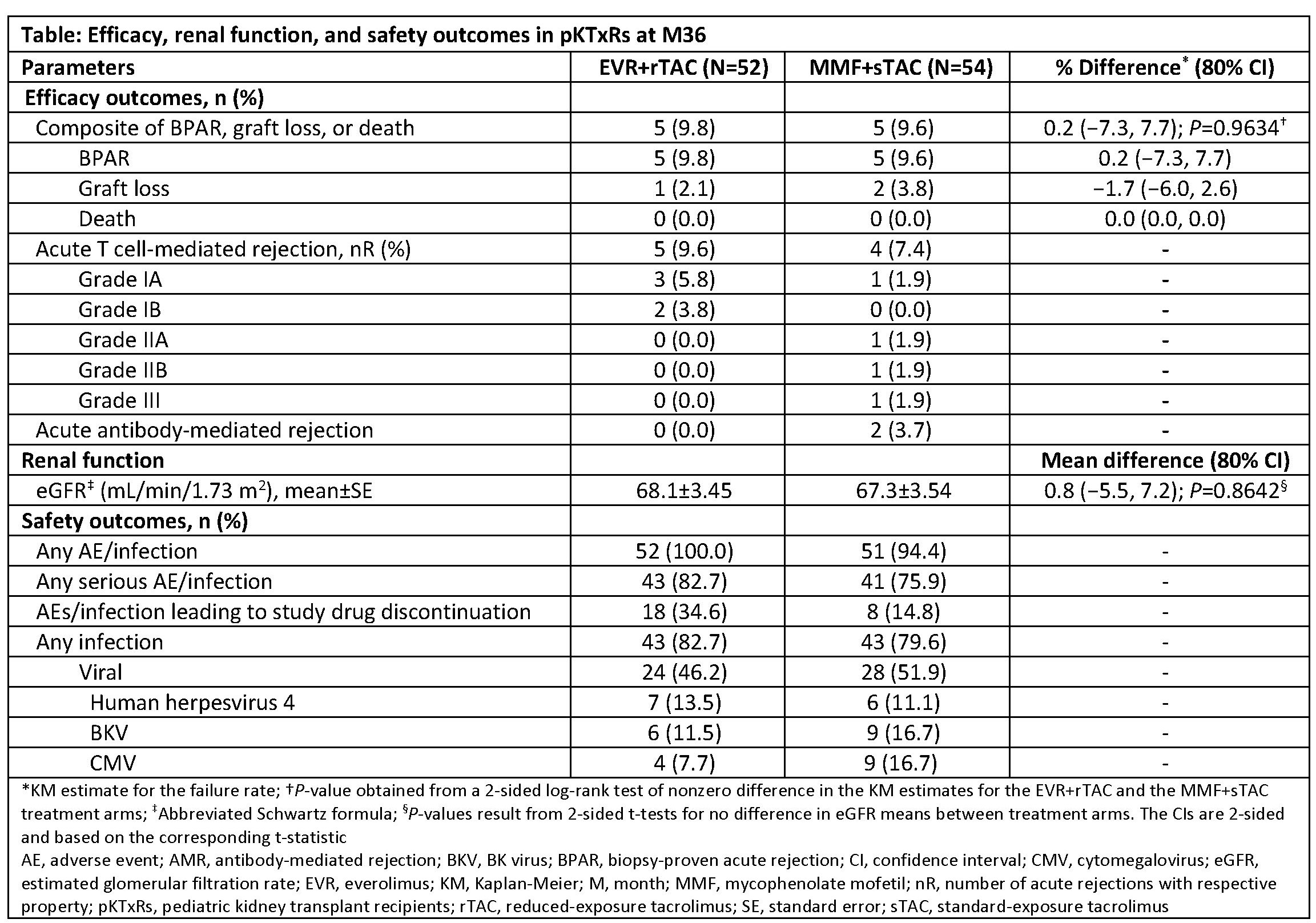
*Results: Overall, 98 (92.5%) patients completed the M36 study (EVR+rTAC, n=47; MMF+sTAC, n=51); drug discontinuation was higher in the EVR+rTAC vs MMF+sTAC arm (42.3% vs 25.9%). Baseline and demographic characteristics were comparable between arms. The mean±SD TAC trough levels were 3.3±1.46 and 6.2±2.53 ng/mL in the EVR+rTAC and MMF+sTAC arms, respectively. Incidence of CEF, mainly driven by BPAR, was comparable between arms with no deaths (Table). Five KTxRs experienced T cell-mediated acute rejection episodes in the EVR+rTAC (grade IA and IB) vs 4 in MMF+sTAC arm (one each of grade IA, IIA, IIB, and III). Mean eGFR at M36 was comparable between arms (P=0.8642). Median changes (Z-score) in height (0.28 vs 0.23) and weight (0.60 vs 0.64) from randomization to M36 were comparable between EVR+rTAC and MMF+sTAC arms. Overall, incidence of adverse events (AEs) and serious AEs was comparable between study arms, with higher drug discontinuation due to AEs in the EVR+rTAC vs MMF+sTAC.
*Conclusions: Despite higher drug discontinuation rate in the EVR+rTAC arm, the outcomes of this trial in pKTxRs showed that conversion to an EVR+rTAC regimen at 4-6 weeks post Tx with an early steroid withdrawal offers comparable efficacy, safety, and renal function vs MMF+sTAC at M36.
To cite this abstract in AMA style:
Silva HTedesco, Ettenger R, Bjerre A, Christian M, Strologo LDello, Marks SD, Pape L, Lopez P, Cousin M, Pandey P, Rauer B, Meier M, Tönshoff B. Effect of Everolimus with Reduced-Exposure Tacrolimus and Early Steroid Elimination in Pediatric Kidney Transplant Recipients: 36-Month Outcomes from the CRADLE Study [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/effect-of-everolimus-with-reduced-exposure-tacrolimus-and-early-steroid-elimination-in-pediatric-kidney-transplant-recipients-36-month-outcomes-from-the-cradle-study/. Accessed February 4, 2026.« Back to 2019 American Transplant Congress