Pretransplant Antibodies Against Angiotensin II Type 1 Receptor in Renal Transplantation: Preliminary Data from the KNOW-KT Study
1Department of Transplantation Surgery, Yonsei University Health System, Seoul, Republic of Korea
2Department of Laboratory Medicine, Yonsei University Health System, Seoul, Republic of Korea
3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea.
Meeting: 2015 American Transplant Congress
Abstract number: 161
Keywords: Alloantibodies, Alloantigens, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Antibodies and Graft Injury: Translational
Session Type: Concurrent Session
Date: Monday, May 4, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 3:03pm-3:15pm
Presentation Time: 3:03pm-3:15pm
Location: Room 121-AB
Background
The antibodies (Abs) against angiotensin II type 1 receptor (AT1R) have been suggested as a risk factor for graft failure and acute rejection. However, incidence and importance of anti-AT1R Abs had not been evaluated in Asia. The aim of our study was to evaluate the incidence of anti-AT1R Abs and their impact on post- transplant outcomes.
Methods
In this multicenter, observational cohort study, we tested anti-AT1R Abs by using AT1R assay kits (One Lambda, CA, USA) in pre-transplant sera from 166 consecutive kidney recipients. A threshold of anti-AT1R antibody levels was statistically determined at 9.05 U/mL based on the time to acute rejection. Serum creatinine and glomerular filtration rates (GFR) at 1 year and were evaluated to compare graft function.
Results
Anti-AT1R Abs were observed in 98/166 (59.0%) of the analyzed recipients. During the 12 month observation period, no graft failure was reported. AT1R (+) patients showed significantly higher incidence of biopsy-proven acute rejection than in AT1R (-) patients (27.6% vs. 10.3%, p=0.007). Multivariate analysis showed that anti-AT1R Abs was an independent risk factor for acute rejection (HR = 3.18, 95% CI = [1.37, 7.39], p=0.007). Mean serum creatinine after 1 year showed a significant difference (1.13 ± 0.34 mg/dL in AT1R (-) vs. 1.27 ± 0.51 mg/dL in AT1R (+), p=0.047). However, there was no significant difference in GFR between groups.
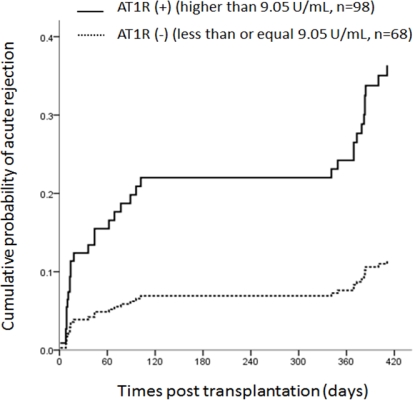 Kaplan-Meier analysis of acute rejection episodes according to pretransplant anti-AT1R Abs
Kaplan-Meier analysis of acute rejection episodes according to pretransplant anti-AT1R Abs
Patients with anti-AT1R Abs > 9.05 U/mL have an increased risk of developing an acute rejection episode during 1 year.
Conclusion
Pretransplant anti-AT1R Abs are associated with acute rejection. Detection of anti-AT1R Abs could be helpful for assessment of immunologic risk for acute rejection.
To cite this abstract in AMA style:
Huh K, Lee J, Park Y, Kim Y, Ahn C. Pretransplant Antibodies Against Angiotensin II Type 1 Receptor in Renal Transplantation: Preliminary Data from the KNOW-KT Study [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/pretransplant-antibodies-against-angiotensin-ii-type-1-receptor-in-renal-transplantation-preliminary-data-from-the-know-kt-study/. Accessed February 25, 2026.« Back to 2015 American Transplant Congress
