Prolonged-Release Tacrolimus Dosing in De Novo Kidney Transplantation: Randomized, Open-Label, Pilot Study
1Asan Medical Center, Seoul, Korea
2Chang Gung Memorial Hospital-Linkou, Taoyuan City, Taiwan
3Samsung Medical Center, Seoul, Korea
4Severance Hospital, Seoul, Korea
5Dongsan Medical Center, Daegu, Korea
6Tri-Service General Hospital, Taipei City, Taiwan
7Astellas Pharma, Inc., Tokyo, Japan
8Astellas Pharma, Inc., Maao, Singapore.
Meeting: 2018 American Transplant Congress
Abstract number: C118
Keywords: Graft survival, Immunosuppression, Kidney transplantation, Renal function
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
A multicenter, randomized, open-label, parallel-group, pilot, 52-wk study in Asian countries that assessed renal function, efficacy, and safety of low vs standard-dose prolonged-release tacrolimus (PRT) in adult kidney transplant recipients (KTRs). Post transplantation, KTRs received PRT from Wks 0–4 (0.2–0.3mg/kg; target tacrolimus trough level 6–10ng/mL). At Wk 4, KTRs were randomized (1:1) to receive PRT as low dose (target 4–6ng/mL Wks 4–12, 3–5ng/mL Wks 12–52) or standard dose (target 6–10ng/mL Wks 4–52). Primary endpoint: estimated glomerular filtration rate (eGFR) over 52 wks. Secondary endpoints (Wk 52) included creatinine clearance (CrCl), serum creatinine (SCr), graft/patient survival, biopsy-confirmed acute rejection (BCAR), composite of graft loss, patient death and BCAR, acute rejection (AR) and steroid-resistant AR. Treatment-emergent adverse events (TEAEs) were recorded. Overall, 66 KTRs received PRT (low n=32; standard n=34) and were analyzed. After per-protocol dose adjustment, mean ±standard deviation tacrolimus trough level was lower with low vs standard-dose PRT (Wk 52, 4.52±1.09 vs 8.04±2.22ng/mL). In the low vs standard-dose group, eGFR was numerically lower early post randomization, but similar at Wks 8–52 (overall least-square-mean difference −2.8; 95% confidence interval −7.9, 2.3; p=0.272). At Wk 52, there was no significant difference in CrCl (p=0.375) or SCr (p=0.547) between groups (Table). All grafts/patients survived; no steroid-resistant AR reported; 4 (12.5%) and 3 (8.8%) patients had AR in the low and standard-dose groups, respectively. Drug-related TEAEs were reported in 34.4% and 38.2% of patients, respectively. Low-dose PRT demonstrated no new safety signals; these pilot data suggest that low-dose PRT was as effective and tolerable as the standard dose for Asian KTRs. 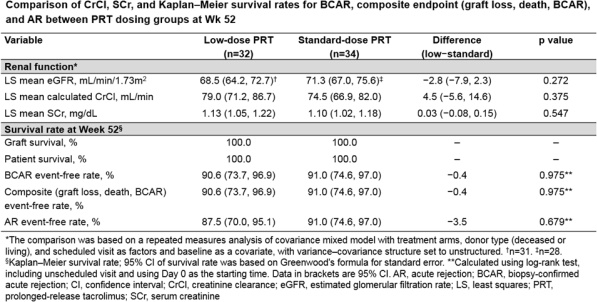
CITATION INFORMATION: Kim Y., Chiang Y., Kim S., Kim M., Park S., Wu S., Horita K., Nakashima Y., Jiang H., Han D. Prolonged-Release Tacrolimus Dosing in De Novo Kidney Transplantation: Randomized, Open-Label, Pilot Study Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Kim Y, Chiang Y, Kim S, Kim M, Park S, Wu S, Horita K, Nakashima Y, Jiang H, Han D. Prolonged-Release Tacrolimus Dosing in De Novo Kidney Transplantation: Randomized, Open-Label, Pilot Study [abstract]. https://atcmeetingabstracts.com/abstract/prolonged-release-tacrolimus-dosing-in-de-novo-kidney-transplantation-randomized-open-label-pilot-study/. Accessed February 24, 2026.« Back to 2018 American Transplant Congress
