Evaluation of Tacrolimus Extended-Release in African American Renal Transplant Recipients
University of Maryland, Baltimore.
Meeting: 2018 American Transplant Congress
Abstract number: C95
Keywords: African-American, Dosage, FK506
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
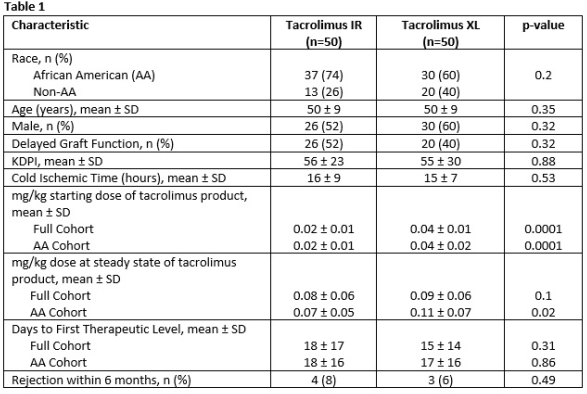
Purpose: Limited data exists to guide appropriate dosing strategies for novel tacrolimus formulations, such as tacrolimus XL (Astagraf XL). This is especially true in the African American (AA) patient population, a group that often requires higher tacrolimus dosing secondary to the more prevalent CYP3A5*1 genotype. We sought to evaluate dosing needs compared to tacrolimus immediate release (IR).
Methods: This was a retrospective, pre- and post-cohort study of deceased donor renal transplants comparing 50 patients who received tacrolimus IR with 50 patients who received tacrolimus XL. All patients received alemtuzumab induction therapy, tacrolimus IR or XL (goal 8-9 ng/mL), mycophenolate 2g/day, and a steroid withdrawal taper. The primary endpoint of interest was the mg/kg dose of tacrolimus required to achieve goal levels at steady state.
Results: Baseline characteristics did not differ between groups. Irrespective of race, patients receiving tacrolimus XL were initiated at a higher weight-based dose of tacrolimus XL; however, this did not impact time to first therapeutic tacrolimus trough (table 1). Notably, the weight-based dose at steady state was significantly higher only for AA patients receiving tacrolimus XL (tac IR 0.07 ± 0.05 vs. tac XL 0.11 ± 0.07, p=0.02).
Conclusion: These data suggest the need for more aggressive dosing in AA recipients receiving tacrolimus XR. This data is in stark contrast of that for Envarsus (tacrolimus ER), in which AA recipients have been shown to require reduced doses compared to tacrolimus IR. Further study is required to determine the impact of these dosing needs on longer term transplant-related outcomes.
CITATION INFORMATION: Sparkes T., Ravichandran B., Williams C., Thomas B., Masters B. Evaluation of Tacrolimus Extended-Release in African American Renal Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Sparkes T, Ravichandran B, Williams C, Thomas B, Masters B. Evaluation of Tacrolimus Extended-Release in African American Renal Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/evaluation-of-tacrolimus-extended-release-in-african-american-renal-transplant-recipients/. Accessed February 20, 2026.« Back to 2018 American Transplant Congress