Everolimus and the Risk of Graft Loss or Death in Kidney Transplant Recipients: A Trial-Based Linkage Study
1Renal Medicine, Royal Prince Alfred Hospital, Camperdown, Australia
2Kidney Node, Charles Perkins Centre, University of Sydney, Sydney, Australia.
Meeting: 2018 American Transplant Congress
Abstract number: C66
Keywords: Graft failure, Immunosuppression, Mortality
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: The use of mTORIs may be associated with a reduction of cancer incidence after kidney transplantation (KT) and improved graft function compared with calcineurin-inhibitor (CNI) based therapy (Rx). Despite these benefits, registry studies and an individual patient data (IPD) meta-analysis have shown an increased risk of death with mTORIs. Using IPD linked to the ANZDATA registry, we assessed the risk of graft loss and death in pts randomised to everolimus (EVL) vs controls (CTL) on standard CNI-based Rx.
Method: We conducted an IPD meta-analysis of all RCTs evaluating EVL versus CTL in Australian and NZ KT recipients between 2001-2012 (Fig 1). IPD was linked with the ANZDATA registry for the outcomes of graft loss and all-cause deaths. We used a random-effects Cox model, to investigate the association between allocation to EVL or CTL and subsequent risk of death-censored graft loss and death.
Results:Five RCTs with 349 pts (EVL=242, Control=107) were included. The RCTs examined EVL using de novo (n=141), early (n=138) and late conversion (n=70) strategies + reduced dose CNI or CNI-withdrawal. CTL consisted of CyA or Tac + MPA or Aza + steroid Rx. The median (IQR) length of follow-up was 9.3 yrs (7.2, 13.5). There were 39 (16%) graft losses and 41 (17%) deaths in EVL vs 12 (11%) graft losses and 13 (12%) deaths in CTL. The proportion of pts who died from cardiovascular disease (CVD) was higher in the EVL (5.4%) vs CTL (0.9%) (p=0.05). Death from other causes were similar (Cancer: 5.8% vs 4.7% p=0.7. Infection: 2.9% vs 2.8% p=1.0). There was no association between EVL and death-censored graft loss (adj HR 1.17 95% CI 0.59 – 2.31 p=0.7) or death (adj HR 1.50 95% CI 0.79 – 2.85 p=0.2).
Conclusion:In this long-term linkage study, EVL was not associated with an increased risk of graft loss or death. A significantly higher proportion of EVL patients died from CVD and further prospective studies are required to confirm this finding. 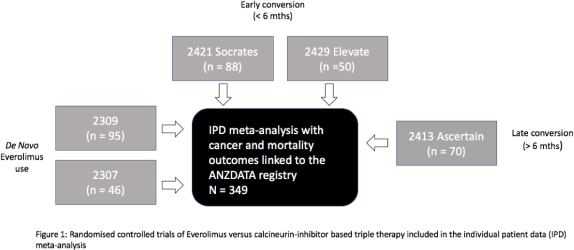
CITATION INFORMATION: Ying T., Wong G., Lim W., Russ G., Pilmore H., Kanellis J., Goodman D., Trevillian P., Campbell S., Suranyi M., Mathew M., Faull R., Masterson R., Walker R., O'Connell P., Chadban S. Everolimus and the Risk of Graft Loss or Death in Kidney Transplant Recipients: A Trial-Based Linkage Study Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Ying T, Wong G, Lim W, Russ G, Pilmore H, Kanellis J, Goodman D, Trevillian P, Campbell S, Suranyi M, Mathew M, Faull R, Masterson R, Walker R, O'Connell P, Chadban S. Everolimus and the Risk of Graft Loss or Death in Kidney Transplant Recipients: A Trial-Based Linkage Study [abstract]. https://atcmeetingabstracts.com/abstract/everolimus-and-the-risk-of-graft-loss-or-death-in-kidney-transplant-recipients-a-trial-based-linkage-study/. Accessed February 20, 2026.« Back to 2018 American Transplant Congress
