De Novo Donor Specific Antibodies in Pediatric Kidney Transplant Recipients Are Highly Responsive to Therapy
Pediatric Nephrology, Lucile Packard Children´s Hospital, Palo Alto, CA.
Meeting: 2018 American Transplant Congress
Abstract number: C33
Keywords: HLA antibodies, Kidney, Pediatric, Rejection
Session Information
Session Name: Poster Session C: Kidney Chronic Antibody Mediated Rejection
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: Pediatric Kidney transplant recipients who develop dnDSAs are at increased risk of allograft loss and rapid decline in GFR. C1q+ DSA, in particular, is associated with highest risk. However, there is no consensus for application of this classification in clinical decision making and little is known about DSA response to therapy.
Methods: Retrospective cohort of 20 pediatric renal transplant patients who formed dnDSAs identified by screening or investigation of allograft dysfunction and received treatment with at least 6 month followup. DSAs were identified by single-antigen flow bead assay. Primary outcome was evolution of DSAs and GFR at 12 months. Secondary outcome was development of proteinuria ≥0.5 g/day. GFR was estimated using Schwartz method. The same pathologists reviewed all biopsies.
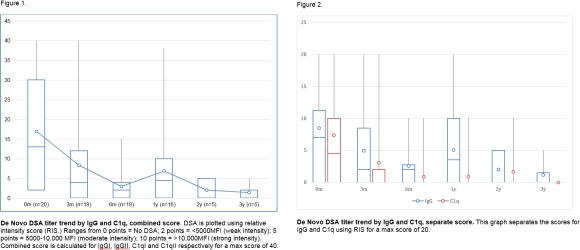
Results: Median followup was 16 months (7-54). At dnDSA diagnosis (table), 11 had allograft dysfunction with GFR decrease >25%. All had IgG dnDSAs, 15 had C1q binding dnDSAs as well. (figure 1.) Resolution of C1q+ DSAs after initiation of therapy was faster than IgG+ DSAs (figure 2). Therapy consisted of intravenous methylprednisolone (n=17), rabbit antithymocyte globulin (n=10), IVIG (n=13), Rituximab (n=13) and 1 received Bortezomib / plasmapheresis. Steroid free immunosuppression was converted to steroid based in 8 patients. 17 had renal allograft biopsy (table 1.) at the time of identification of the dnDSA. GFR did not deteriorate (baseline Median 68(44-223) vs to 66(7-114) at 12 months (p=0.3.)). During the followup period, one patient with persistent C1q had allograft failure and 6 had new onset proteinuria.
Conclusion: De Novo DSA were highly responsive to therapy. C1q DSAs depleted faster than IgG DSAs. Most patients had mixed ABMR and TCMR at diagnosis. Allograft function was stable at 12 months.
CITATION INFORMATION: Sigurjonsdottir V., Grimm P. De Novo Donor Specific Antibodies in Pediatric Kidney Transplant Recipients Are Highly Responsive to Therapy Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Sigurjonsdottir V, Grimm P. De Novo Donor Specific Antibodies in Pediatric Kidney Transplant Recipients Are Highly Responsive to Therapy [abstract]. https://atcmeetingabstracts.com/abstract/de-novo-donor-specific-antibodies-in-pediatric-kidney-transplant-recipients-are-highly-responsive-to-therapy/. Accessed March 9, 2026.« Back to 2018 American Transplant Congress