Efficacy and Safety of Immediate- and Prolonged-Release Tacrolimus in De Novo Pediatric Transplantation: Randomized Study
1University Hospital Motol, Prague, Czech Republic
2Osp Pediatrico Bambino Ges[ugrave], Rome, Italy
3King's College Hospital, London, United Kingdom
4The Children's Memorial Health Institute, Warsaw, Poland
5Manchester University Foundation Trust, Manchester, United Kingdom
6Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom
7APHP-Hôpital Universitaire Necker, Paris, France
8Alder Hey Children's Hospital, Liverpool, United Kingdom
9Université
Lyon 1 et Hospices Civils de Lyon, Lyon, France
10Birmingham Children's Hospital, Birmingham, United Kingdom
11Astellas Pharma Europe Ltd, Chertsey, United Kingdom.
Meeting: 2018 American Transplant Congress
Abstract number: B294
Keywords: Efficacy, Immunosuppression, Pediatric, Safety
Session Information
Session Name: Poster Session B: Liver: Pediatrics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
This study assessed long-term efficacy/safety of prolonged-release tacrolimus (PR-T) vs immediate-release tacrolimus (IR-T) in de novo pediatric transplant pts. Phase II, parallel-group, multicenter, open-label study in de novo kidney, liver or heart allograft recipients aged <16 years. Pts randomized (1:1) to receive once-daily PR-T or twice-daily IR-T (initial daily dose 0.075mg/kg in heart, 0.3mg/kg in liver/kidney recipients). Target tacrolimus trough level 5–15ng/mL from Day 22. Pts followed-up to Year 1 post randomization. Pts remained on immunosuppressive regimen they were receiving on entry. Efficacy endpoints: biopsy-confirmed acute rejection (BCAR), pt/graft survival, efficacy failure. Adverse events (AEs) were recorded. Overall, 44 pts (20 PR-T; 24 IR-T; mean ±standard deviation [SD] age 10.6±3.1 yrs) received study drug. Baseline characteristics were similar between treatment groups. Mean±SD tacrolimus daily dose was 0.3±0.1mg/kg initially and 0.2±0.1mg/kg at Year 1 with both PR-T and IR-T; at Year 1, tacrolimus trough levels were 6.6±2.2 and 5.4±1.6ng/mL, respectively; BCAR in 1 and 4 pts, respectively. No graft losses/deaths. Efficacy failure occurred in 8 pts (1 PR-T; 7 IR-T). AEs were experienced by most pts, were mild/moderate in 73.8%, and led to study discontinuation in 1 pt (IR-T) (Table). No new safety signals reported. These data support long-term use of PR-T-based immunosuppression in de novo pediatric solid organ allograft recipients. 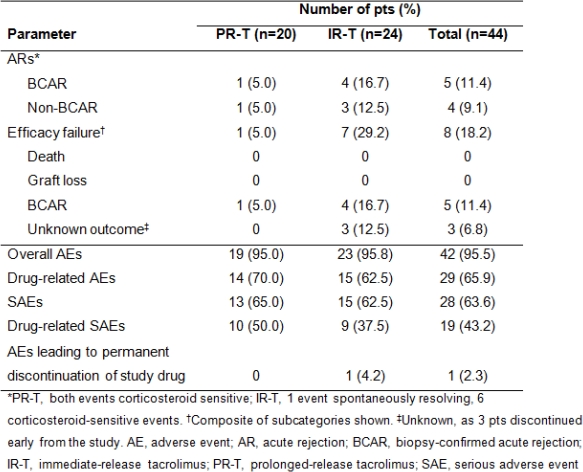
CITATION INFORMATION: Vondrak K., Parisi F., Dhawan A., Grenda R., Webb N., Marks S., Debray D., Holt R., Lachaux A., Kelly D., Kazeem G., Undre N. Efficacy and Safety of Immediate- and Prolonged-Release Tacrolimus in De Novo Pediatric Transplantation: Randomized Study Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Vondrak K, Parisi F, Dhawan A, Grenda R, Webb N, Marks S, Debray D, Holt R, Lachaux A, Kelly D, Kazeem G, Undre N. Efficacy and Safety of Immediate- and Prolonged-Release Tacrolimus in De Novo Pediatric Transplantation: Randomized Study [abstract]. https://atcmeetingabstracts.com/abstract/efficacy-and-safety-of-immediate-and-prolonged-release-tacrolimus-in-de-novo-pediatric-transplantation-randomized-study/. Accessed February 12, 2026.« Back to 2018 American Transplant Congress
