Long-Term Efficacy and Safety of Prolonged-Release Tacrolimus in Stable Pediatric Allograft Recipients Converted from Immediate-Release Tacrolimus
1Astellas Pharma Europe Ltd, Chertsey, United Kingdom
2Children's Memorial Health Institute, Warsaw, Poland
3APHP-Hôpital Universitaire Necker, Paris, France
4Birmingham Children's Hospital, Birmingham, United Kingdom
5University Hospital Necker Enfants Malades, Paris, France
6Manchester University Foundation Trust, Manchester, United Kingdom
7University Hospital Motol, Prague, Czech Republic
8Hôpital Femme-Mère-Enfant, Bron Cedex, France
9ISMETT-IRCCSS, Palermo, Italy
10University Children's Hospital, Heidelberg, Germany
11Hospital Papa Giovanni XXIII, Bergamo, Italy
12Great Ormond Street Hospital for Children, London, United Kingdom
13Cliniques Universitaires Saint-Luc UCL, Brussels, Belgium.
Meeting: 2018 American Transplant Congress
Abstract number: B217
Keywords: Efficacy, Immunosuppression, Pediatric, Safety
Session Information
Session Name: Poster Session B: Kidney: Pediatrics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Study assessed long-term efficacy/safety of prolonged-release tacrolimus (PR-T) in stable solid organ allograft recipients (aged 5–16 yrs) who participated in a multicenter, Phase II pharmacokinetics study of one-way conversion from twice-daily immediate-release tacrolimus (IR-T) to once-daily PR-T. Pts continued to receive their IR-T-based regimen on Days 1–7. Day 8, pts converted from IR-T to PR-T (1:1mg total daily dose). Exposure to tacrolimus was similar for IR-T (Day 7) and PR-T (Day 14). During 12-month follow-up, efficacy endpoints were biopsy-confirmed acute rejection (BCAR), pt/graft survival, efficacy failure. Secondary endpoint: safety (adverse events [AEs]). Overall, 79 pts (kidney, n=48; liver, n=29; heart, n=2) were included (mean ±standard deviation [SD] age 11.6±2.8 yrs). Mean±SD tacrolimus daily dose was 0.10±0.05 and 0.09±0.05mg/kg at baseline and Month 12, respectively; tacrolimus trough levels were 5.1±1.9 and 5.2±2.1ng/mL. BCAR was reported in one kidney transplant recipient. Pt/graft survival was 100%. AEs were experienced by 67 (84.8%) pts, were mild in 56.7%, and led to study discontinuation in one pt (Table). No new safety issues were reported. The data support long-term PR-T-based immunosuppression use in stable pediatric transplant pts converted from IR-T. 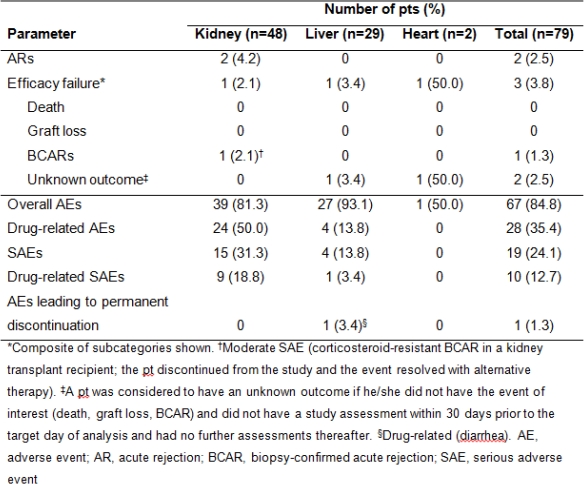
CITATION INFORMATION: Undre N., Rubik J., Debray D., Kelly D., Iserin F., Webb N., Czubkowski P., Vondrak K., Sellier-Leclerc A-.L., Riva S., Tönshoff B., D'Antiga L., Marks S., Reding R., Kazeem G. Long-Term Efficacy and Safety of Prolonged-Release Tacrolimus in Stable Pediatric Allograft Recipients Converted from Immediate-Release Tacrolimus Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Undre N, Rubik J, Debray D, Kelly D, Iserin F, Webb N, Czubkowski P, Vondrak K, Sellier-Leclerc A-L, Riva S, Tönshoff B, D'Antiga L, Marks S, Reding R, Kazeem G. Long-Term Efficacy and Safety of Prolonged-Release Tacrolimus in Stable Pediatric Allograft Recipients Converted from Immediate-Release Tacrolimus [abstract]. https://atcmeetingabstracts.com/abstract/long-term-efficacy-and-safety-of-prolonged-release-tacrolimus-in-stable-pediatric-allograft-recipients-converted-from-immediate-release-tacrolimus/. Accessed March 3, 2026.« Back to 2018 American Transplant Congress
