Safety and Efficacy of Rabbit Anti-Thymocyte Globulin Induction in Pediatric Patients Undergoing Cardiac Transplantation
1Pharmacy, Medical University of South Carolina, Charleston, SC
2Cardiac Transplant, Medical University of South Carolina, Charleston, SC.
Meeting: 2018 American Transplant Congress
Abstract number: B53
Keywords: Heart transplant patients, Induction therapy, Pediatric
Session Information
Session Name: Poster Session B: Heart and VADs: All Topics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Purpose: Limited data are available in pediatric cardiac transplant patients that focus on the safety and efficacy of rabbit anti-thymocyte globulin (rATG) induction therapy. The aim of this study was to evaluate the use of rATG induction therapy in pediatric patients undergoing cardiac transplantation.
Methods: A retrospective, single-center, cohort study was conducted at a large academic medical institution. Patients were included if they underwent cardiac transplantation by the pediatric cardiac transplant team at the institution between January 1, 1998 and July 1, 2017. The primary objective was to determine the rate of overall graft loss in patients receiving rATG induction therapy compared with those without rATG induction therapy. Secondary objectives included rate of acute rejection, time to first rejection, development of donor specific antibodies (DSA), development of transplant coronary artery disease (TCAD), and safety of rATG induction compared with no rATG induction.
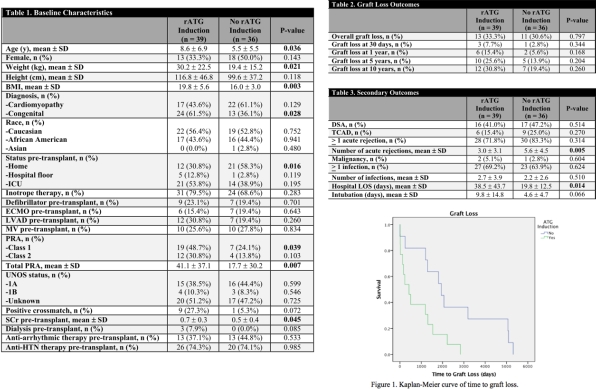
Results: A total of 75 patients were identified between January 1, 1998 and July 1, 2017. Thirty-nine patients who received rATG induction and 36 patients without rATG induction were included in the analysis. On average, patients who received rATG induction were older and had higher panel reactive antibody and baseline serum creatinine (Table 1). There was no significant difference in overall graft loss, however there was a significant difference in time to graft loss as seen in Figure 1 (P = 0.014). There were a significantly greater number of acute rejection episodes in patients who did not receive rATG (P = 0.005), but no significant differences in the number of infections or development of a malignancy, TCAD, or DSAs.
Conclusion: Patients who received rATG induction therapy were at higher risk of rejection at baseline. The use of rATG was not associated with a higher overall risk of graft loss, infection, or malignancy but did reduce the number of acute rejections suffered by pediatric patients post-cardiac transplantation.
CITATION INFORMATION: Sell M., Haney A., Sprott K., Burnette A., Henderson H., Savage A. Safety and Efficacy of Rabbit Anti-Thymocyte Globulin Induction in Pediatric Patients Undergoing Cardiac Transplantation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Sell M, Haney A, Sprott K, Burnette A, Henderson H, Savage A. Safety and Efficacy of Rabbit Anti-Thymocyte Globulin Induction in Pediatric Patients Undergoing Cardiac Transplantation [abstract]. https://atcmeetingabstracts.com/abstract/safety-and-efficacy-of-rabbit-anti-thymocyte-globulin-induction-in-pediatric-patients-undergoing-cardiac-transplantation/. Accessed February 16, 2026.« Back to 2018 American Transplant Congress