Outcomes after HIV+ Liver Transplant: A HOPE in Action Study
1JHU, Baltimore
2Mount Sinai, New York
3NIH, Bethesda
4UCSF, San Francisco
5UMN, Minneapolis.
Meeting: 2018 American Transplant Congress
Abstract number: 567
Keywords: HIV virus, Liver transplantation, Outcome
Session Information
Session Name: Concurrent Session: New Frontiers in HIV and Hepatitis
Session Type: Concurrent Session
Date: Tuesday, June 5, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:06pm-5:18pm
Presentation Time: 5:06pm-5:18pm
Location: Room 4C-3
The HOPE in Action Pilot Trial (NCT02602262) is assessing the safety and feasibility of HIV+ deceased donor (D+) kidney and liver transplantation for HIV+ recipients (R+) in the United States pursuant to the HIV Organ Policy Equity Act.
METHODS: We compared post-transplant outcomes between liver transplant (LT) recipients of HIV D+ organs and HIV-uninfected donor (D-) organs. Characteristics were compared using Wilcoxon rank sum and Fischer's exact tests; rejection was measured as time-to-event.
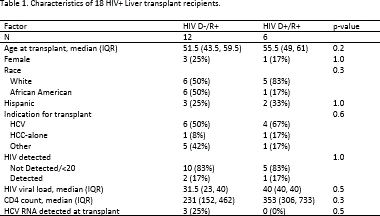
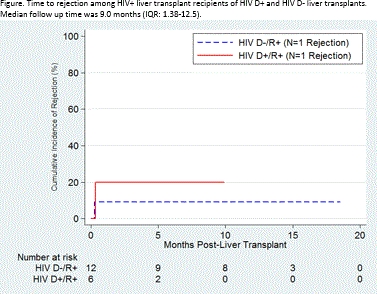
RESULTS: Between 3/2016 and 11/2017, 18 HIV R+ liver transplants (LT) were performed at 5 centers: 6 from HIV D+, 12 from HIV D-, including 5 livers from donors with false-positive HIV screening tests, confirmed later to be negative. HIV D+/R+ and HIV D-/R+ LT recipients were similar in age, sex, race, HIV RNA, CD4 count, and HCV RNA (p-values>0.05, Table 1). One death occurred 9 days post-HIV D+/R+ transplant due to aspiration pneumonia deemed unrelated to the donor HIV status. There were no graft failures. There was 1 AIDS-defining infection in the HIV D-/R+ arm. No HIV-virologic failures occurred in either group. Median follow up time was 9.0 months (IQR: 1.38-12.5). Rejection was similar between groups (1 rejection in each arm representing 8.3% of HIVD-/R+ and 16.7% of HIVD+/R+, p=0.6).
CONCLUSION: We report the first HIV D+/R+ liver transplants in the United States since implementation of the HOPE Act. These cases provide proof-of-concept that the use of HIV+ and HIV false-positive donors can improve access to organ transplants for HIV+ candidates with high waitlist mortality.
CITATION INFORMATION: Durand C., Bowring M., Tobian A., Brown D., Cameron A., Ottman S., Oshima K., Ostrander D., Redd A., Huprikar S., Haydel B., Lerner S., Florman S., Chandran S., Chao A., Kirchner V., Pruett T., Massie A., Segev D. Outcomes after HIV+ Liver Transplant: A HOPE in Action Study Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Durand C, Bowring M, Tobian A, Brown D, Cameron A, Ottman S, Oshima K, Ostrander D, Redd A, Huprikar S, Haydel B, Lerner S, Florman S, Chandran S, Chao A, Kirchner V, Pruett T, Massie A, Segev D. Outcomes after HIV+ Liver Transplant: A HOPE in Action Study [abstract]. https://atcmeetingabstracts.com/abstract/outcomes-after-hiv-liver-transplant-a-hope-in-action-study/. Accessed January 28, 2026.« Back to 2018 American Transplant Congress