Meeting OPTN Requirements for Living Kidney Donation Follow-Up: Findings from the KDOC Study
1Beth Israel Deaconess Medical Center, Boston
2Cleveland Clinic, Cleveland
3Rhode Island Hospital, Providence
4Maine Medical Center, Portland
5Erie County Medical Center, Buffalo
6University of Iowa, Iowa City
7Mayo Clinic, Scottsdale
8University of Wisconsin, Madison.
Meeting: 2018 American Transplant Congress
Abstract number: 529
Keywords: Donation, Multicenter studies, Public policy
Session Information
Session Name: Concurrent Session: Kidney Living Donation: Programmatic Issues
Session Type: Concurrent Session
Date: Tuesday, June 5, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Room 6B
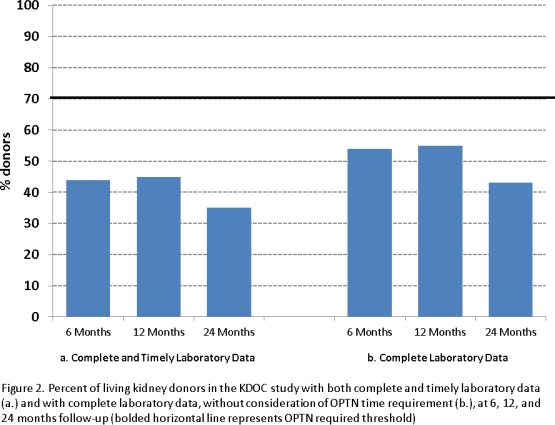
The Organ Procurement and Transplantation Network (OPTN) requires programs to submit complete, accurate and timely clinical information for 80% and laboratory data for 70% of living kidney donors (LKDs) at 6-, 12-, and 24-month follow-up. A program is noncompliant with policy for a time-point if any required clinical/laboratory element is missing or not collected ±60 days of the time-point. We retrospectively analyzed data collected in the NIH-funded Kidney Donor Outcomes Cohort (KDOC) study to ascertain whether policy requirements were met. Complete and timely clinical information was collected for 61% (6 months), 52% (12 months), and 48% (24 months) of LKDs; complete and timely laboratory data were collected for 44% (6 months), 45% (12 months), and 35% (24 months) of LKDs. Higher rates of data collection were observed when removing the ±60 day timing requirement. None of the six KDOC programs met policy requirements at any of the follow-up periods for clinical information, while two programs met requirements at both the 6- and 12-month follow-up periods for laboratory data. Despite substantial resources and efforts, we were unable to meet policy requirements and this experience is generally consistent with what others have reported and what has been reported by the OPTN.
CITATION INFORMATION: Rodrigue J., Sokas C., Fleishman A., Schold J., Morrissey P., Whiting J., Vella J., Kayler L., Katz D., Jones J., Kaplan B., Pavlakis M., Mandelbrot D. Meeting OPTN Requirements for Living Kidney Donation Follow-Up: Findings from the KDOC Study Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Rodrigue J, Sokas C, Fleishman A, Schold J, Morrissey P, Whiting J, Vella J, Kayler L, Katz D, Jones J, Kaplan B, Pavlakis M, Mandelbrot D. Meeting OPTN Requirements for Living Kidney Donation Follow-Up: Findings from the KDOC Study [abstract]. https://atcmeetingabstracts.com/abstract/meeting-optn-requirements-for-living-kidney-donation-follow-up-findings-from-the-kdoc-study/. Accessed January 5, 2026.« Back to 2018 American Transplant Congress