A Protocol for the Management of Alemtuzumab Induced Leukopenia in Kidney Transplant Recipients
University of Maryland Medical Center, Baltimore.
Meeting: 2018 American Transplant Congress
Abstract number: B149
Keywords: Kidney transplantation, Neutropenia
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Purpose: Leukopenia is a common complication following alemtuzumab induction in kidney transplant recipients (KTR), often limiting its use. Our institution developed a protocol to manage this complication (Table 1). We sought to determine the incidence of leukopenia following alemtuzumab in KTR and to characterize the management of leukopenia and neutropenia per this protocol.
| Table 1. | |
| WBC/ANC | Intervention |
| WBC ≤ 2.5 x 103/microL | Check CMV PCR
Hold SMX/TMP and valganciclovir (if not CMV mismatch) Order ANC |
| ANC < 1000/mm3 | Decrease mycophenolic acid product by 50% if CNI therapeutic
Administer G-CSF |
| ANC < 500/mm3 | Hold mycophenolic acid product
Administer G-CSF Initiate low dose prednisone if CNI sub-therapeutic and POD <60 or high risk for rejection |
Methods: This was an IRB approved, single center, retrospective study of adult KTR between 11/2015 – 02/2017 who received alemtuzumab. We assessed baseline demographics, G-CSF administration, medication changes, white blood cell (WBC) count (leukopenia defined as WBC<4×103/microL; severe leukopenia defined as WBC≤2.5×103/microL) and absolute neutrophil count (ANC) (neutropenia defined as ANC≤1000/mm3) at specified time points until 10 weeks post-transplant.
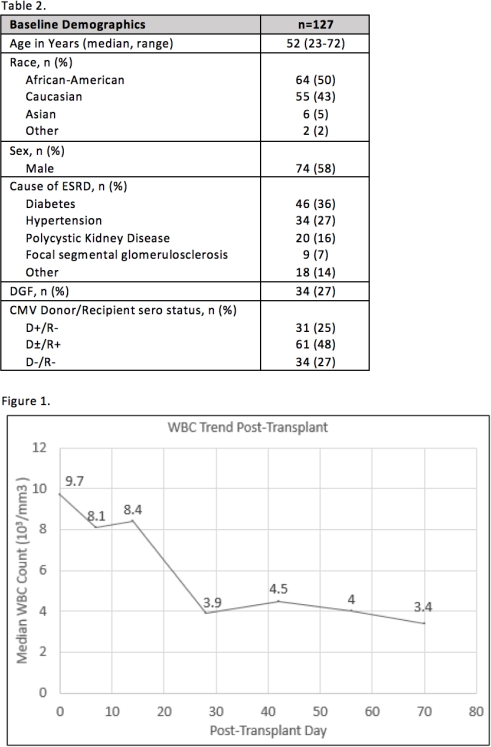
Results: During the study period, 98 KTR (77%) experienced leukopenia and 54 KTR (43%) developed severe leukopenia. Of the KTR with severe leukopenia, 3 required only medication changes and 19 required G-CSF after medication changes to resolve their severe leukopenia. Ten KTR received G-CSF outside the parameters of the protocol. In total, 29 KTR received G-CSF and 25 KTR had no intervention for leukopenia. The median WBC for all patients declined from 9.7×103/microL immediately post transplant to 3.4×103/microL at the completion of followup (Figure 1).
Conclusion: Our study confirmed that centers should anticipate more than 40% of KTR receiving alemtuzumab will experience severe leukopenia. Providers, however, may take different approaches to the management of this complication. Our center's protocol offers a stepwise approach to the management of leukopenia in KTR who received alemtuzumab for induction.
CITATION INFORMATION: Hammad S., Ravichandran B., Crist M., Masters B. A Protocol for the Management of Alemtuzumab Induced Leukopenia in Kidney Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Hammad S, Ravichandran B, Crist M, Masters B. A Protocol for the Management of Alemtuzumab Induced Leukopenia in Kidney Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/a-protocol-for-the-management-of-alemtuzumab-induced-leukopenia-in-kidney-transplant-recipients/. Accessed February 18, 2026.« Back to 2018 American Transplant Congress