Ciprofloxacin for BK Viremia Prophylaxis in Kidney Transplant Recipients: Results of a Prospective, Randomized Controlled Trial
S. Patel, S. Kuten, R. Knight, J. Nolte, D. Nguyen, E. Graviss, L. Moore, I. Agboli, C. Pham, A. Gaber.
Houston Methodist Hospital, Houston, TX.
Meeting: 2018 American Transplant Congress
Abstract number: 135
Keywords: Infection, Kidney transplantation, Polyma virus
Session Information
Session Name: Concurrent Session: Kidney Transplant Goes Viral
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:18pm-5:30pm
Presentation Time: 5:18pm-5:30pm
Location: Room 606/607
Fluoroquinolone (FQ) antibiotics have in vitro activity against BK virus, with several reports indicating they may reduce the risk of BK infection in kidney transplant recipients (KTRs). Only a single prospective trial exists, which has demonstrated failure of levofloxacin to prevent BK viruria in KTRs. Herein, we describe the results of a prospective, double-blind, placebo-controlled trial evaluating ciprofloxacin (CIP) for prevention of BK viremia (BKV).
Methods. Two hundred KTRs were randomized in a 2:1 ratio to receive CIP 500 mg/day (n=133) or matching placebo (n=67) for 3 months starting within the first week post-transplant. The primary endpoint was BKV incidence, detected by PCR at 6 months post-transplant. Secondary endpoints included time to BKV, viral load, BK nephropathy, bacterial infections, FQ resistance, rejection and graft loss.
Results. The two groups were similar with respect to recipient, donor, and immunosuppressant characteristics with the exception of a greater proportion of black donors (21% vs. 6%; p=0.01) and prednisone maintenance use (95% vs. 87%; p=0.04) in the CIP vs. placebo group, respectively.. Study results are shown in Table 1. BKV occurred in 24 (18%) CIP patients and 4 (6%) placebo patients (p=0.02). No differences were seen in initial viral load, time to BKV, or BK nephropathy. No differences were seen in the rate of positive urine or blood cultures, total number of positive cultures, or rate of C. difficile. CIP administration was associated with a significantly higher rate of FQ resistance (83% vs. 50%; p=0.04), however no other differences in adverse events occurred.
Conclusion. A 3-month course of CIP 500 mg/day post-transplant did not prevent BKV at 6 months, nor was it associated with any secondary benefits. This study further reinforces the inability of FQ antibiotics to prevent BK in a prospective, controlled setting.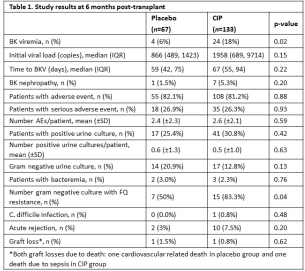
CITATION INFORMATION: Patel S., Kuten S., Knight R., Nolte J., Nguyen D., Graviss E., Moore L., Agboli I., Pham C., Gaber A. Ciprofloxacin for BK Viremia Prophylaxis in Kidney Transplant Recipients: Results of a Prospective, Randomized Controlled Trial Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Patel S, Kuten S, Knight R, Nolte J, Nguyen D, Graviss E, Moore L, Agboli I, Pham C, Gaber A. Ciprofloxacin for BK Viremia Prophylaxis in Kidney Transplant Recipients: Results of a Prospective, Randomized Controlled Trial [abstract]. https://atcmeetingabstracts.com/abstract/ciprofloxacin-for-bk-viremia-prophylaxis-in-kidney-transplant-recipients-results-of-a-prospective-randomized-controlled-trial/. Accessed January 29, 2026.« Back to 2018 American Transplant Congress
