Genetically Modified Porcine Erythrocytes: A Cell Model for Studying Human Preformed Antibody-Dependent Xenoimmune Responses In Vitro
Surgery, University of Alabama at Birmingham, Birmingham, AL.
Meeting: 2018 American Transplant Congress
Abstract number: 94
Keywords: Antibodies, knockout, Pig, Xenotransplantation
Session Information
Session Name: Concurrent Session: Xenotransplantation
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:30pm-3:42pm
Presentation Time: 3:30pm-3:42pm
Location: Room 602/603/604
Background:
Powerful gene editing technology is a promising approach to provide genetically-modified pigs as donors for clinical cell, tissue and organ xenotransplantation. Prior to starting a wide-scale clinical application of xenotransplantation, the humoral immune reject remains an important obstacle to be overcome. In the current study, we assessed human preformed antibody-dependent xenoimmune response in vitro to porcine erythrocytes from genetically-modified pigs.
Methods:
Erythrocytes were isolated from Wild type and genetically-modified pigs, including GGTA1/ β4GalNT2 knockout (DKO) and GGTA1/CMAH/β4GalNT2knockout (TKO), as well as health persons. Commercial health human serum were purchased. The levels of human antibody binding (IgG/IgM) to the cells were analyzed using Agglutination assay and Flow cytometry. Antibody-dependent cell phagocytosis was assessed by ImageStreamX Mark II Imaging flow cytometry, and Confocal Image. 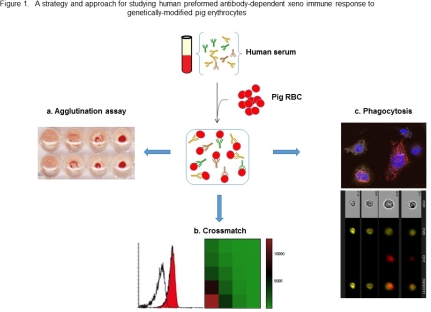
Results:
Carbohydrate antigens of genetically-modified erythrocytes were analyzed using Isolectin GS-IB4, DBA, and anti Neu5Gc antibody. Hemagglutination levels of TKO RBC were significantly reduced compared with that DKO RBC. Flow cytometry crossmatch shows that human IgG/IgM binding to TKO RBC was significantly decreased compared to that to DKO RBC. Both confocal image and flow cytometry imaging confirmed that opsonized RBC was phagocytosed by human THP1-derived macrophages. Flow cytometry data shows that phagocytosis rates for TKO RBC was 5-fold lower compared with WT RBC and 1.8-fold lower compared with DKO RBC by THP1-derived macrophages.
Conclusions:
Removing carbohydrate antigens markedly prevents human preformed antibody-dependent xenoimmune responses to erythrocytes from genetically-modified pigs,The results suggest that the gene editing approach may be able reduce antigenicity in pig cells and organ tissues to levels that enable the xenotransplantation humoral barrier to be overcome.
Grant Support: United Therapeutics Corp. provided funding for some of this study.
CITATION INFORMATION: Wang Z., Martens G., Ladowski J., Tector M., Tector A. Genetically Modified Porcine Erythrocytes: A Cell Model for Studying Human Preformed Antibody-Dependent Xenoimmune Responses In Vitro Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Wang Z, Martens G, Ladowski J, Tector M, Tector A. Genetically Modified Porcine Erythrocytes: A Cell Model for Studying Human Preformed Antibody-Dependent Xenoimmune Responses In Vitro [abstract]. https://atcmeetingabstracts.com/abstract/genetically-modified-porcine-erythrocytes-a-cell-model-for-studying-human-preformed-antibody-dependent-xenoimmune-responses-in-vitro/. Accessed February 23, 2026.« Back to 2018 American Transplant Congress
